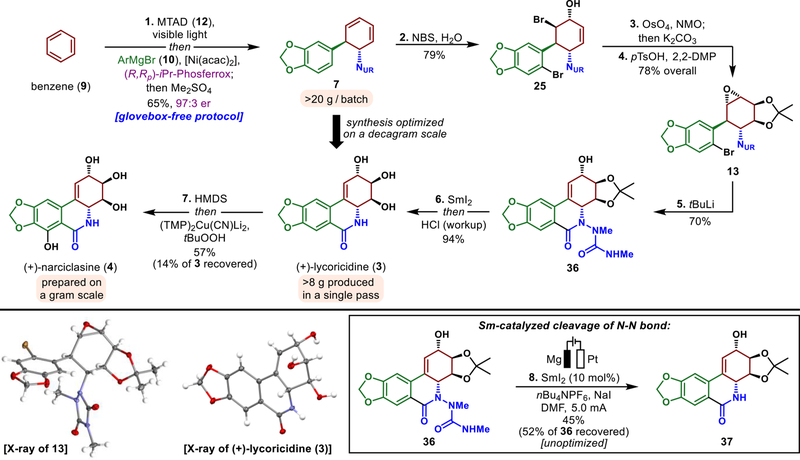
Figure 8.
Synthesis of (+)-lycoricidine (3) and (+)-narciclasine (4). Reagents and conditions: 1. benzene (9), MTAD (12), CH2Cl2, visible light, −78 °C; then [Ni(acac)2] (1.5 mol %), (R,Rp)-iPr-Phosferrox (2.0 mol %), Grignard reagent 10, CH2Cl2, THF, −78 to +25 °C; then Me2SO4, K2CO3, 65% (97:3 er); 2. NBS, H2O, THF, 25 °C, 79%; 3. OsO4 (5 mol %), NMO, citric acid, acetone, H2O, tBuOH, 25 °C; then K2CO3, 25 °C; 4. 2,2-dimethoxypropane, pTsOH (10 mol %), CH2Cl2, 25 °C, 78% over two steps; 5. tBuLi, THF, −78 °C, 70%; 6. SmI2, MeOH, 0 °C, then HCl, 0 °C, 94%; 7. HMDS, TFA (1.0 mol %), MeCN, 25 °C; then solvent removal and (TMP)2Cu(CN)Li2, THF, −78 → 0 °C; then tBuOOH, THF, −78 °C; acidic workup, 57% (14% of 3 recovered); 8. NaI, nBu4NPF6, SmI2 (10 mol %), DMF, 25 °C, Mg anode, Pt cathode, 5.0 mA, 45% (52% of 36 recovered).