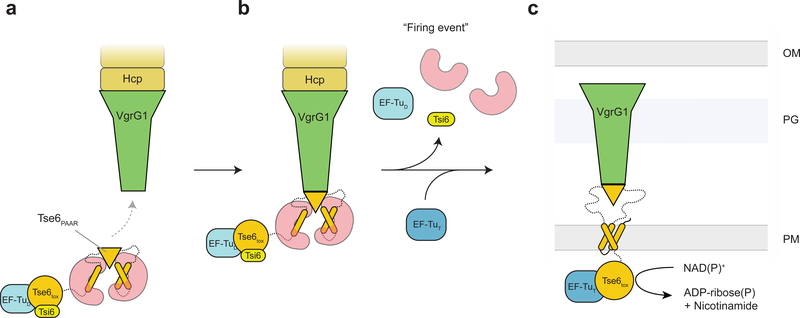
Figure 6. Model for Tse6 effector loading and delivery.
(a) EagT6 (red) binds to the hydrophobic TMDs of Tse6 (orange). This prevents the protein from aggregating and ensures the correct folding of the PAAR domain. The PAAR domain specifically recognizes VgrG1 and mediates the loading of Tse6 onto VgrG1. EagT6 is therefore crucial for the efficient assembly of the Tse6-VgrG1 complex. The binding of EF-Tu (light blue) and Tsi6 (yellow) completes the T6S effector/chaperone complex. (b) Prior to firing EagT6, EF-Tu and Tsi6 dissociate from the complex activating the Tse6toxin. (c) The T6SS punctures the outer membrane of the target cell, forcefully bringing Tse6 into the periplasm. Tse6 spontaneously enters the inner membrane and translocates the Tse6toxin domain across the membrane. On the cytosolic side of the membrane, Tse6toxin binds to EF-Tu and acts as glycohydrolase depleting the cytosolic NAD(P)+ pool. OM – outer membrane, PG – peptidoglycan, IM – inner membrane, D – donor, T – target.