Abstract
Although diabetes is a nationwide epidemic, US Latinos are a particularly vulnerable population. Culturally appropriate interventions can combat this disparity, especially those that increase social support. However, these interventions face significant cost and time barriers, which mHealth (mobile health) may overcome. This trial examines the benefit of adding social support to an existing text-message based, patient-focused mHealth intervention for emergency department patients with poorly controlled diabetes. Family members and friends of patients were randomized to mHealth augmented social support training (daily text-messages that synchronize with the patient messages) or a pamphlet based training (the same content mailed to their house.) We hypothesize that patients who received mHealth augmented social support will have a larger improvement in diabetes management (glycosylated hemoglobin or A1C) than those receiving standard support at six-months, and that improvement will be sustained at twelve-months. Secondary patient outcomes are clinical (weight, blood pressure), behavioral (medication adherence, self-care activities) and psychosocial (general and diabetes-specific social support, self-efficacy, diabetes-related distress, depression, fatalism and quality of life). We screened 2004 patients and enrolled 166 patient/supporter dyads. 70% of patients are Spanish-speaking, 51% female, with a mean A1C of 10.8. We employed innovative measures to remotely enroll family members and support a bilingual population, which will assist other investigators in design of similar trials. The findings of our trial will have real-world applicability for clinicians, health system administrators, health educators and mHealth developers who aim to improve the health of this vulnerable population.
Keywords: Diabetes, Latinos, mHealth, emergency department, social support
1. Introduction:
Although diabetes is a nationwide epidemic, US Latinos are a particularly vulnerable population. Latinos are more likely than non-Latino Whites to develop diabetes and have higher rates of diabetic complications and diabetes related mortality.1,2 This disparity is prominent in Los Angeles County, where almost half the residents are Latino, and diabetes prevalence is higher than the national average.3,4 Patient with diabetes who rely on the Los Angeles County + University of Southern California Emergency Department for care are a high need population, with poor glycemic control, low diabetes knowledge, and limited access to care.5 These patients are not often adequately served by the current healthcare system, and require dedicated attention to address their unique health needs and barriers to care.
Culturally and linguistically appropriate interventions can combat this disparity, especially social support interventions. Training family members and peers to support patients with diabetes has been shown to improve patient motivation, healthy behaviors and glycemic control,6–10 and is accepted by Latino populations.11–13 However, traditional social support interventions require in-person training for family and friends, as well as physical space and personnel for training. Additionally, support training is often provided to the most proximate people to the patient with available time, rather than the most influential person identified by the patient. In-person training also limits scalability in populations with limited transportation and financial resources. Strategies to decrease these barriers are needed; mobile health (mHealth) may be a solution.
mHealth is the use of mobile phones to provide public health and medical solutions. mHealth interventions improve disease management of chronic illnesses, including diabetes.14–17 However, existing app-based mHealth interventions require smartphones, which are not widely used in resource-poor communities such as safety-net emergency departments (EDs).18 However, Latino populations have high rates of mobile phone ownership, text-message use, and access health information via mobile phones more frequently that other digital sources.19,20 Latino patients from low-resource settings have enthusiastically joined text-message and interactive voice-recording interventions to improve diabetes self-care.21–23
ED care is an opportunity to reach patients and families during a health crisis, when they are susceptible to behavior change.24 As emergency care is more expensive than healthcare in other sites,25 ED interventions should maximize the benefit of these visits through improved behaviors and health outcomes. ED-based interventions can also bridge patients until primary care can be established for those lacking primary care, or during the wait for the next visit for patients with limited access to a primary care provider.
In this paper, we describe the protocol and enrollment for the randomized controlled trial TExT-MED+FANS (Trial to Examine Text-Messaging in Emergency patients with Diabetes+ Family and friends Network Support), adding a mobile social support module, FANS to the TExT-MED intervention to provide emotional context and highly personal touch. TExT-MED+FANS uses mHealth to overcome the transportation and time obstacles that social support solutions face by offering social support training via a mobile platform. Using mobile training, a patient can select the most influential person to support them, rather than the most proximate.
2. Methods:
2.1. Study Design and Aims
In this twelve-month, comparative effectiveness randomized controlled trial (enrollment period July 2017 to October 2018), ED patients with poorly-controlled diabetes mellitus from an urban, safety net medical center were randomized to one of two arms: [1] an mHealth intervention for self-management education augmented by mHealth augmented social support (TExT-MED+FANS) or [2] the same patient-oriented mHealth intervention with minimally augmented social support (TExT-MED + pamphlet). The aims are of the study are:
Aim 1: Compare effectiveness of TExT-MED+FANS to TExT-MED + pamphlet on glycemic control (A1C) among emergency department patients with diabetes at the end of the six month intervention and after a six-month washout phase.
Hypothesis
A text message module (FANS: (Family And friend Network Supporters) delivered to a patient’s supporter will improve A1C at six months compared to that of patients whose supporters receive similar support information by mail.
A related exploratory aim will examine potential moderators of intervention effectiveness in lower A1C (gender, health literacy, baseline social support, baseline mobile technology use, psychosocial wellbeing and supporter distress related to diabetes) as well as mediators of intervention effectiveness assessed at three, six, nine and twelve months (change in perceived social support, supporter distress related to diabetes, change in mobile technology use, and self-report of healthy behaviors).
Aim 2: Compare effectiveness of TExT-MED+FANS to TExT-MED+pamphlet on diabetes related clinical, behaviors and psychosocial well being among emergency department patients with diabetes at the end of the intervention and after six-month post intervention phase.
Aim 3: Evaluate experience with TExT-MED+FANS and impact on perceptions and motivation We will conduct a qualitative analysis of patient and supporter experience with TExT-MED+FANS through individual semi-structured interviews.
2.2. Comparative Effectiveness Randomized Controlled Trial
2.2.1. Patient recruitment
Patients were recruited from July 2017 to October 2018. IRB approval for this study was obtained prior to study initiation from the USC Health Sciences Institutional Review Board. This study enrolled ED patients with both type I and type II diabetes at LAC+USC (Los Angeles County + University of Southern California Medical Center). ED patients with diabetes in this healthcare system have been found to have poor glycemic control, poor diabetes specific knowledge, and poor access to primary care.5
2.2.2. Screening, Eligibility Criteria and Recruitment
Trained research assistants (RA) conducted screening and enrollment during the daytime and evening hours in the LAC+USC ED. They surveyed the ED electronic patient tracking system for patients with diabetes. Inclusion and exclusion criteria are listed in Table 1. Only subjects with A1C ≥8.5% were enrolled in this trial, as these patients have the greatest need for intervention and potential to demonstrate beneficial intervention effect. The A1C-based eligibility requirement was verified in the emergency department during the patient’s visit, using the Afinion AS-100 capillary point-of-care A1C meter (Axis-Shield PoC AS, Oslo, Norway). Patients who reported both type 1 and 2 diabetes were enrolled, as prior work with this population has shown that 30% of patients are unsure which type of diabetes they have.5 RAs explained the purpose of the study and obtained written informed consent in the language of the patient’s preference. To be eligible, patients had to identify a family member or friend to agree to serve as a supporter. Patients were aware at enrollment that the designated supporter could receive multiple text-messages per day, and would be prompted to offer increased support.
Table 1:
Eligibility criteria for participants
| Exclusion Criteria |
| Psychiatric involuntary hold, or in police custody |
| Altered mental status |
| Clinically unstable to consent and complete baseline assessment |
| Inclusion Criteria |
| Age 18 or greater |
| Stable ownership of mobile phone |
| Able to send and receive text messages |
| Reads English or Spanish |
| A1C 8.5 or greater |
| Identifies a support person who can be contacted within 2 weeks to enroll |
2.2.3. Enrollment & Randomization
The goal of this investigation is to study augmenting existing social support via mHealth, so patients were only eligible to be enrolled in the study if a supporter agreed to participate as well; however, this took up to three weeks to contact and enroll the supporter. Given the potential lag, all potentially eligible patients were registered in the SHERPA platform used by Agile Health while in the ED. Occasionally, patients lacked cellular service in the ED and were not able to text-in the Federal Communications Commission-required YES message to opt in during their initial ED visit; we texted and called these patients daily for up to one week until they texted back in YES. The supporter was not contacted until after the patient was fully registered in the system. If a supporter was not eventually enrolled as well, the patient still received the patient text-messaging program, however they were removed from further participation in the study.
Supporters were enrolled either in the ED during the initial contact with the patient or remotely by telephone if they were not present in the ED. Each patient was instructed to rank up to three family or friends who would provide them the most support. As the intervention encourages communication between a patient and a supporter, only one supporter was enrolled per patient to not overwhelm patients with suggestions for healthy living from multiple loved ones. We collected multiple contact numbers for each potential supporter from each patient. We called daily for up to three weeks to enroll supporters.
Enrolling supporters consisted of verbal consent, confirming age>18 and the ability to send and receive text messages, completion of supporter survey instruments and registration in the SHERPA platform. Registration in the SHERPA system required a YES text back from the supporter. If the supporter did not respond with the required text-in YES message, we called them daily to remind and assist them with completing registration. After supporter enrollment was completed, the dyads were randomized to the FANS mHealth-augmented social support or pamphlet-based social support education for the supporter. All patients received the TExT-MED patient program.
2.3. Patient Intervention – TExT-MED
The original TExT-MED curriculum description and development are previously described.21 TExT-MED was developed from National Diabetes Education Program (NDEP)26 messages adapted to the character constraints of text messages (160 characters); messages emphasized behavior change.27 It was a six-month, fully automated, text message based program designed to increase knowledge, self-efficacy and subsequent disease management and glycemic control. The twice-daily text messages for patients consisted of: 1) educational/motivational messages 2) medication reminders 3) diabetes trivia questions and 4) healthy living challenges. After the original TExT-MED study was completed, the program was purchased, modified and commercialized by Agile Health into a new, enhanced program called MyAgileLife. MyAgileLife, consists of three messages a day and has a greater focus on behavioral skills than the original intervention TExT-MED– including setting goals, tracking progress, enabling social support, creating environmental cues, and celebrating success. MyAgileLife also contains messages designed to increase engagement by including trivia questions and patient self-assessments of motivation and disease management which request a response. For the patient curriculum in this study, for technical issues related to the timing of the messages we used a slightly locally modified version of the MyAgileLife program.
2.4. FANS Family member/Supporter Intervention
2.2. FANS Curriculum Development
The FANS support messages were initially developed from National Diabetes Education Program and American Diabetes Association (ADA) recommendations for family members to offer emotional, informational, and instrumental support. The existing patient curriculum of text messages was reviewed, and of the 3 daily patient text messages, 2 messages were selected to develop a coordinating supporter FANS text message. FANS text messages were then translated into Spanish by a native Spanish speaker, and back translated by a native Spanish speaker of a different origin country. Four family members of patients with diabetes, all bilingual native Spanish speakers, then reviewed all English and Spanish FANS and TExT-MED patient messages to confirm retention of meaning. The messages were also tested and refined with a group of Spanish-speaking promatoras—community health workers with special training in health education who are valued opinion leaders in their neighborhood. The promatoras identified messages that needed further development. In particular, they noted translations that had negative connotations or nuances. For example, a message in English stated, “to get things in order”; the Spanish translation of “sigue en linea” reminded some promotoras of current political discourse regarding waiting for one’s turn to cross the border. Due to the potential negative connotations, we were careful to reword such messages.
2.4.2. Theoretical basis
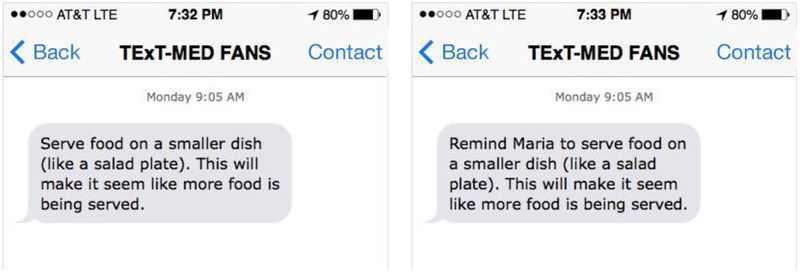
The FANS messages for supporters mirror the patient messages, and the coordinating messages are sent to the patient and the supporter synchronously (see Figure 1). This synchronous message delivery is designed to instigate conversation between the patient and the supporter, increasing the “stickiness” of the message. The FANS messages are constructed on a model of four arenas of social support: 1) Instrumental support (tangible goods and actions), 2) Informational support (knowledge), 3) Emotional support and 4) Appraisal support (feedback regarding accuracy of beliefs and appropriateness of actions).28 Currently, the NDEP and American Diabetes Association (ADA) recommend a combination of emotional, informational, and instrumental support from loved ones. Given the financial constraints of many of the patients and family members of this safety-net ED population, the FANS messages emphasize emotional, informational and non-financial forms of instrumental support.
Figure 1:
Example of patient message and corresponding family member message
2.4.3. FANS Supporter Curriculum and Delivery
The FANS (Family and friend Network Support) curriculum consists of six months of twice daily messages for the enrolled supporters and synchronizes in time and content with the patient messages. The messages are 160 characters in length or less, to conform to short-message-service text message requirements. A few messages require that two separate texts be sent to encompass the entire content. The messages are designed to allow personalization with the supporter’s name, or the patient’s name (see Table 2 for example), as this was a requested feature in prior qualitative evaluation of mHealth user experience.29 Most messages were informational, however approximately one FANS message per week was a support challenge message that encouraged contact with the patient, prompted the supporter to engage in a specific care behavior or challenged the FANS perform the same health behavior the patient was challenged to do, and to communicate that effort to the patient. In total, the FANS curriculum consists of 381 messages of educational and motivational content with an emphasis on inspiring appropriate social support. 39% of messages focus on informational support, 42% of messages focus on emotional support and 18% focus on instrumental support.
Table 2:
Example FANS messages from each support domain
| Support domain | Emotional | Informational | Instrumental |
|---|---|---|---|
| Example FANS message from support person curriculum | Celebrate every time John does something for their health. Feel great about your progress together. | The A1C test (A-one-C) shows what Jackie’s blood sugar has been over the last 3 months. The A1C goal for most people is 7. | Challenge: Learn the names and doses of Gio’s medications. Write them down and keep the list handy for health appointments |
2.5. Efforts to support Non-English speaking and bilingual dyads.
In order to support Spanish and English speaking patient and supporters, we created versions of the intervention in each language. Additionally, we allowed patients and supporters to select different languages, as patients and supporters may have different language preferences for texting. This required an additional layer of programming in the platform to synchronize messages between dyads. As an additional step to support Spanish-speaking participants required all research staff using Spanish with patients were required to pass a language certification test.
2.6. Participant Safety
There are two areas of risk to patients in this intervention. The first is the risk of hypoglycemia as patients improve their medication adherence, as we anticipate that up to half of patients will be on insulin or oral insulin secretagogues. Patient knowledge of symptoms and treatment for hypoglycemia is low, and is the first focus of educational messages sent to both supporters and patients. Additionally, on enrollment, patients were instructed to report episodes of hypoglycemia to the research team (either by text or voice message) and to call their primary doctor and to inquire about medication adjustment. If the patient did not have a regular primary care doctor, the research team instructed the patient to visit the urgent access center at LAC+USC where a safety-net system exists for patients lacking a medical home. A clinical pharmacologist working with a family medicine specialist conducts same day/next day appointments to make medication adjustments for diabetes, hypertension and anticoagulation medications. We will evaluate for a difference in patient reported hypoglycemic events between the two groups at three, six and twelve months. Another risk associated with this intervention involves safe texting habits. All patients and supporters were reminded not to text while driving, operating heavy machinery, while crossing streets or in any other situation in which they need to be aware of their surroundings. There may be an increase in phone bills due to the number of texts. Participants were advised that there may be increased costs. Our group has previously reported that more than 90% of our target patient population has unlimited text plans, so we anticipate this to be a minor risk.18
2.7. Study measures, data collection procedures and schedule
2.7.1. Data collection procedures and schedule
Patient assessments occur at enrollment, three, six, nine and twelve months on behavioral and psychosocial outcomes, and at baseline, six and twelve months for clinical outcomes. Potential intervention effect mediators and modifiers are collected at each time point. Trained RAs conduct in-person assessments using standardized protocols and equipment, in the language of the patient’s preference. All RAs that speak with Spanish with patients complete a language competency assessment prior to interacting with patients in Spanish. For assessments at three and nine months, participants have the option of an in-person, mail or phone appointment.
Supporter assessments occur at baseline, six and twelve months. Supporters have the option of an in-person, mail or phone appointment. All assessments are with trained RAs in the language of the participant’s preference.
To ensure data integrity, all RAs are trained by a research coordinator and directly observed for adherence to standard survey administration protocols. The Afinion AS100 point of care machine undergoes manufacturer recommended maintenance and quality control checks with standardized controls. All data is directly entered in real-time into REDCap,30 so that transcription errors are minimized.
2.7.2. Primary and secondary outcome measures
The primary outcome is patient change in glycemic control measured by change in hemoglobin A1C at six months post-randomization. RAs will collect point-of-care values from an Afinion AS100 capillary point of care machine.
Secondary outcomes are changes in clinical, behavioral and psychosocial. Clinical outcomes are weight, BMI, blood pressure. Participants are weighed on the same scale at enrollment and follow up assessments, without shoes or overclothes. Blood pressure is measured by study RAs after the patient is seated for 5 minutes, with the average of the second and third reading recorded. Healthy behaviors are measured by the Summary of Diabetes Self-care Activities31 and medication adherence will be measured by the Wilson 3 item Medication Adherence scale.32 We measure healthcare utilization by the patients’ report of clinic appointments, ED visits and hospitalizations. This self-report will be confirmed by a manual review of the electronic medical record. Psycho-social factors of self-efficacy (Diabetes Empowerment Scale Short Form),33 diabetes related distress (Diabetes Distress Scale),34 depression (PHQ-9),35 fatalism (Diabetes Fatalism Scale)36 and quality of life (World Health Organization WHO-5 Well Being Index)37 will be collected at baseline and follow up assessments at three, six, nine and twelve months after randomization.
2.7.3. Potential effect modifiers and mediators
We are collecting information on several potential mediators and modifiers of the intervention on patients’ glycemic control. Potential modifiers at baseline include mobile technology use and health literacy. Mobile technology use will be measured by questions modeled after the Pew Hispanic Center survey19 with the addition of questions about frequency of contact between the patient and supporter, and the proportion of communication that is about diabetes. Health literacy will be measured by the questions developed by Chew, et al.38
Potential social support mediators of the intervention will be measured as baseline and at follow-ups. Diabetes-related supportive and obstructive family behaviors are measured by patient report on the Diabetes Family Behavior checklist.39 Diabetes-specific social support is measured by the Diabetes Care Profile Support Questions,40 while general social support is captured by the Norbeck Social Support Questionnaire Emotional and Tangible subscales.41 Supporter diabetes-related distress is measured by response to the Partner Distress Scale42, which was initially developed for partners of patients with type 1 diabetes, but is used in this expanded support context with permission of the scale author. Supporter mobile usage is captured with the Pew questions. Frequency of patient-supporter contact and proportion of communication that is about diabetes will be confirmed by collecting this information from the supporter as well.
Engagement with the intervention will be measured by percentage of quizzes and assessments that patients and supporters have responded to at the end of the six-month intervention. These responses are tracked by the SHERPA platform.
2.8. Qualitative assessment of user experience and impact on patient perceptions and motivations
To fully understand the impact that TExT-MED+FANS has on patient self-efficacy, motivations and behavior, we are performing a qualitative analysis of TExT-MED+FANS employing semi-structured interviews with patients and supporters from the intervention arm as they complete the trial. Through this qualitative analysis, we explore potential mechanisms that contribute to changes in behaviors and outcomes, including changes to self-efficacy, perceived barriers, perceived threat and severity of diabetes and perceived benefits of glycemic control. Additionally, we examine the user experience of both patients and supporters, focusing on frequency and content of messages.
Patients and supporters will be interviewed in their primary language. In the case of bilingual patients, they will participate in the language in which they received their text messages. A natively fluent Spanish-speaking RA will conduct the Spanish language interviews. We will conduct at least 60 interviews (30 with intervention group patients and 30 with family member/supporters) with the goal of achieving saturation of coding themes during analysis. If necessary, more interviews may be performed to achieve saturation. Interviews will be audio recorded to accurately capture both the words and the context of statements made by participants. Recordings will be transcribed in the original language, then reviewed and corrected a research coordinator. Spanish language transcriptions will be translated by native Spanish speakers.
2.9. Retention Efforts
An ED population is more transient and can be more difficult to follow up with than clinic based populations; additionally, this trial recruited from primarily low-income patients whose jobs often do not allow phone calls on duty, and work irregular hours. To increase completion of over-the-phone surveys and remind patients of their in-person appointments, systematic efforts are made. Patients are texted through the text-messaging platform and called on their primary and back up phone number. If the patient does not respond, we contact the supporters directly by texting and phone calls. We collect an alternative phone number to contact the patient (i.e another family member or close friend) so that patients receive reminders about study appointments from multiple sources.
“Opting out” of the intervention only requires a single text of “STOP”, so we confirm each of these messages with patients and supporter to be sure that the opt-out was intentional. As this is an intention to treat analysis, patients are still followed at six and twelve months, even if they did not receive the full six months of messages.
2.10. Statistical Analysis
2.10.1. Primary and Secondary Outcomes
The primary outcome in this trial is the change in A1C from baseline to six-months and six to twelve months, with the exposure of interest of TExT-MED+FANS. Normality of the outcome variable (six-month change) will be graphically evaluated. We will employ longitudinal methods with a mixed effects regression model to account for correlated outcome data (zero-six month and six-twelve month changes) and loss to follow up. Analyses will be conducted by intent-to-treat, with participants analyzed according to their randomized intervention regardless of adherence. Because change in A1C is the outcome, participants who drop out prior to six-months will not be included in the analysis. All participants who complete the twelve-month study will provide two outcome measures of six-month change: a zero-six month measure of treatment efficacy, and a six-twelve month measure of sustainability of treatment effect. The linear mixed effects model will include a random intercept term for participants. Fixed effects will include treatment allocation, initial level of A1C (zero month measure for treatment efficacy, six-month measure for sustainability), and a covariate of study period (zero-six month, six-twelve month). The main effect of treatment will test for group differences over both zero-six and six-twelve month periods. An interaction term of treatment by study period will test for differences in treatment effects by study period; treatment effects will be estimated and tested for differences by study period in this interaction model. Model assumptions including normality of model residuals and homogeneity of variance will be evaluated. A sensitivity analysis confined to adherent participants (those who have not opted out of messages and have received 75% or greater of messages confirmed by message delivery platform) will be conducted. Mixed effects linear regression models will be conducted on secondary outcomes as detailed above.
2.10.2. Planned Subgroup Analysis:
To determine if subgroups of participants are differentially affected by the intervention, secondary analyses evaluating intervention moderators are planned. For A1C and each of the secondary outcomes, interaction terms (randomized intervention-by-moderator product terms) will be added to the mixed effects linear models described above. Variables evaluated as moderators will include gender, language preference, years with diabetes, baseline frequency of mobile usage, physically proximity to supporter, baseline social connectedness, and baseline support. If any of these factors indicate significant moderation, intervention effects will be estimated by levels of the moderator. Secondary analyses of the primary A1C outcome will use structural equation modeling to evaluate the secondary behavior and efficacy outcomes as mediators of the TEXT-MED+FANS intervention. Initial analyses will evaluate the associations of changes in behavior and efficacy variables with change in A1C using mixed effects models as detailed above. The A1C mediating model will then include randomized intervention, baseline A1C, any moderating variables detected above, and change in a behavior or efficacy outcome as a possible mediator. Mediation will be tested with bootstrapped samples, evaluating the direct and indirect (mediated) effects of changes in behavior and efficacy outcomes.43,44 The final model will include significant moderators and mediators of the TEXT-MED+FANS effect on A1C.
Additionally, after completion of enrollment, substantial differences in baseline support and contact between patients and their selected supporters became evident, as some supporters took up to three weeks to enroll. We now plan on conducting a sub-analysis based on supporter immediate availability for enrollment versus delayed enrollment.
2.10.3. Qualitative Analysis
The analysis of the interview is transcript-based to enhance rigor. The study team will develop an initial set of codes after the first reading of the transcripts. This coding scheme will be further developed through the analysis process. Themes will be open coded into overarching phenomenon categories as per standard thematic analysis/grounded theory techniques and then be reanalyzed and selectively coded into categories that will comprise the experience with TExT-MED+FANS.45 As further participant quotations are coded and analyzed, concepts and phenomena are compared to one another to ensure the coding structure continue to accurately represent the experiences of the patients. The transcripts will be analyzed using Dedoose™ to organize, tally and investigate the themes identified. Dedoose™ is a web-based qualitative analysis tool that contains data organization tools necessary to complete an analysis of different subgroups.46 Additionally, Dedoose™ contains a correlation feature that will be critical in ensuring accurate and consistent coding between investigators. This process enhances reliability of the coding procedure, which is critical, as at least two researchers will code the transcripts. Disagreements will be discussed with study investigators and consensus achieved. Strategies to maintain integrity, including consistent use of the discussion guide, audio-taping, independent professional transcription, use of researchers with diverse backgrounds for data analysis, standardized coding and analysis will be used to enhance validity of the qualitative findings.
Qualitative data will be combined with the quantitative results to gain a better understanding of which patients benefited the most from the intervention. Patients will be coded as “high responders” (A1C drop of 1 or greater) or “low responders.” By entering this quantitative information into Dedoose™, we will be able to organize the quotes and corresponding themes into these two categories. By re-examining the quotes and themes with this dichotomous grouping, patterns of motivational and behavioral change may become more evident.
2.11. Sample size
We enrolled 166 patient-supporter dyads, which assuming a 30% loss to follow up, gives a sample size of 116 total dyads. With power of 0.8 and alpha of 0.5, using the standard deviation of final A1C of 1.6, (the value of our prior trials), this will give this trial the ability to detect A1C difference of 0.84 between the two groups at six month follow up.
3. Results
3.1. Screening and Recruitment
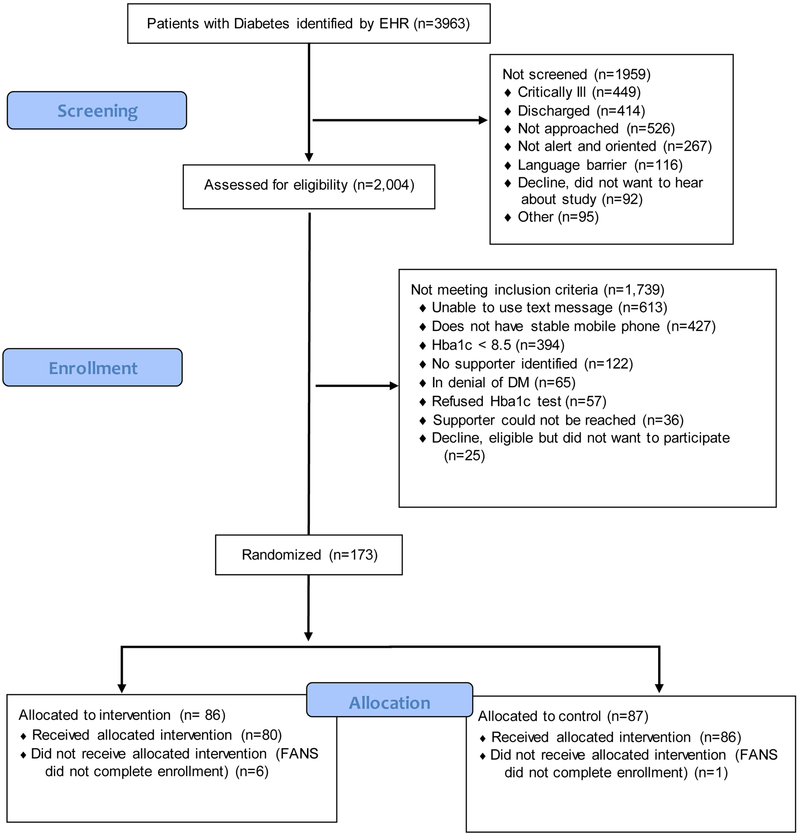
RAs identified nearly 4000 ED patients with diabetes via electronic medical record real-time searches. Over half of these patients (2004) were screened for eligibility (see Figure 2 for CONSORT style diagram of screening and enrollment for reasons for ineligibility). Of the 2004 patients screened for eligibility, 173 (9%) met criteria and agreed to enroll. The most common reason to not meet eligibility was not using text-messages at baseline (31%, 613/2004 patients), followed by not having a stable mobile phone number (21%, 427/2004 patients). A substantial portion of patients had A1C levels below the threshold (20%, 394/2004 patients.) Less than 10% of patients could not identify a potential supporter or identified a potential supporter who could not be reached. Of note, 65 patients (3% of screened patients) did not believe they had diabetes, despite having been diagnosed by a physician. After randomization, 7 supporters failed to complete the initial process of enrollment in the study (i.e. initially answered call from RA, agreed to participate, but then ended call without completing assessment and did not answer any further calls or texts.) We ended enrollment once the final cohort of 166 patient-supporters dyads was reached.
Figure 2:
Screening and Enrollment of patients into TExT-MED+FANS
3.2. Participant characteristics
The enrolled patient cohort is 51% female, 70% Spanish-speaking, 79% born outside US. The mean age of enrolled patients is 47.2 years. The mean A1C is 10.8. Patients self-reported the type of diabetes, with 67% (111) reporting type II diabetes (111), 8% (14) reporting type I diabetes and 25% (41) who did not know which type of diabetes they had. Of these 166 patients, 50% (83) used insulin at enrollment and 10% (16) were managed with diet and exercise without medications.
3.3. Supporter characteristics
The supporters are 70% female and 57% Spanish-speaking with 66% born outside the US. Their mean age is 43.7 years. Of these supporters, 20% (33) also had diabetes; 68% (23) with type II diabetes, 6% (2) with type I DM, and 26% (8) who did not know the type of diabetes they had. The supporters are predominantly family members: 31% are spouses (51), 14% are siblings (24), 23% are an adult child of the patient (39), 16% are other relatives (28), 12% are friends (20) and 4% of patients did not wish to disclose the nature of their relationship with their supporter.
4. Discussion
We have designed the TExT-MED+FANS investigation and enrolled a full cohort of patients and supporters into the study. This text-message based intervention is designed to improve existing social support and reinforce behavior changes that will lead to improved glycemic control for low-income ED patients with diabetes. The primary outcomes of intervention efficacy at six-months and sustainability at twelve months will be reported at trial conclusion. This investigation will provide evidence for adding social support components to existing mHealth interventions, and will clarify the mechanism of improved social support on disease-specific psychosocial and behavioral mediators of diabetes management. This trial is innovative in two ways: 1) patients who access emergency services for care of chronic diseases are understudied and present unique challenges to successful clinical research and 2) the remote enrollment of a family member or friend into a social support curriculum. Additionally, by using multiple time points for collection of potential mediators, we are able to use mixed-effects models which better account for missing data which can be a challenge in ED-based studies. These features presented unique challenges and required new solutions which may assist other investigators in the design of their trials.
TExT-MED+FANS is unique in that ED patients with diabetes are enrolled with a loved one, capitalizing on existing support relationships and augmenting the support already provided. This contrasts with work in which patient were paired with previously unknown peers or lay supporters,7,10,47–49 which have been positively received, had moderate impact on intentions and behaviors, but showed heterogenous results on glycemic control. Latino populations have shown promise with these interventions, potentially due to the importance of family in health decisions and disease management for many Latinos. Prior studies have included patients who were unable to recruit a family member to serve as a supporter, which complicates findings of the role of social support in intervention efficacy on improving diabetes self-management.50,51 In these studies, less than half of participants ended up with a supporter enrolled.
Approximately 10% of our patients were excluded due to inability to identify or enroll a support person. By only randomizing after we confirmed supporter availability, we better isolated the role of improved social support on disease management. Our focus on inner city, ED patients from a hospital that primarily serves Latino patients improves our knowledge of a vulnerable population. The focus on ED patients with poorly controlled diabetes rather than patients from more common primary care venues allows us to reach patients who do not have the benefit of regular medical attention. Patients without a medical home are more likely to seek care in the ED, and have worse glycemic control that those with regular access to primary care.52–55 The ED patients with poorly controlled diabetes in this trial are not adequately managed within the current healthcare system. Their health may be particularly sensitive to the social relationships we are augmenting.
The patients in this study have previously been underrepresented in mHealth and diabetes research, as few studies are done with predominantly Spanish-speaking populations in the United States.9,10,56 70% of our patients preferred Spanish, while 57% of supporters preferred Spanish. The few diabetes mHealth interventions for Spanish-speaking populations have reduced symptoms of depression, improved diabetes self-management and glycemic control and reduced ED utilization.21–23 To support Spanish and English speaking patient and supporters, as well as bilingual dyads, we created a system that allowed for patients and supporters to individually select their language preference. Requiring all research staff using Spanish to pass a language certification test also ensured that the intervention provided adequate support to Spanish-speaking participants. The additional resources required to support multiple languages in this intervention were significant. However, as more research shows this to be efficacious and cost-effective for Spanish-speaking populations, health systems will be encouraged to pursue these tools for all patients.
The remote consenting and enrollment of supporters in the synchronized social support curriculum is crucial in engaging this high need population. As loved ones of safety net ED patients may not have the available time off from work or transportation for formal enrollment visits, the use of telephone consent and guidance through the mHealth platform allowed for a broader group of patients to be enrolled. In fact, we failed to enroll few eligible patients due to inability to enroll their designated supporter. Some patient designated a supporter who was immediately available, while other FANS required several phone calls to enroll in the trial; this could represent a difference in baseline social condition. To look at the potential difference this intervention could have in these situations, we now plan to analyze the difference in the intervention looking at immediate vs. delayed supporter enrollment. The effectiveness of the intervention in these two situations will impact potential feasibility of scaling up the intervention to population levels.
In summary, we designed a mHealth intervention that activates existing social support to improve diabetes-related health behaviors and subsequent disease management and glycemic control. Important features of this interventions are dual language availability, randomization to mHealth augmented versus traditional paper-based curriculum social support only after confirming loved one availability and recruiting from an ED that serves a safety-net population. These factors created a need for specialized protocols to ensure that this vulnerable population is represented in mHealth research for diabetes self-care, which may assist other investigators with their study design. As our intervention concludes, the findings of this investigation will direct future social support work in diabetes self-management as well as mHealth interventions for low-resource populations.
5. Acknowledgements
This work was supported by NIH K23DK106538 and the University of Southern California Undergraduate Research Assistant Program. Data entry via REDCAP is supported by grants UL1TR001855 and UL1TR000130 from the National Center for Advancing Translational Science (NCATS) of the U.S. National Institutes of Health. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. We would like to thank Raquel Carla Martinez for her assistance in developing figures and editing.
Abbreviations:
- ED
Emergency Department
- A1C
glycosylated hemoglobin A1C
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Elizabeth Burner, Department of Emergency Medicine, Keck School of Medicine of the University of Southern California.
Janisse Mercado, Department of Emergency Medicine, Keck School of Medicine of the University of Southern California.
Antonio Saenz Hernandez, Keck School of Medicine of the University of Southern California.
Anne Peters, Division of Endocrinology, Keck School of Medicine of the University of Southern California.
Lourdes Baezconde-Garbanati, Department of Preventive Medicine, Keck School of Medicine of the University of Southern California.
Sanjay Arora, Department of Emergency Medicine, Keck School of Medicine of the University of Southern California.
Shinyi Wu, School of Social Work, University of Southern California.
Works cited
- 1.Geiss LS, Wang J, Cheng YJ, et al. Prevalence and incidence trends for diagnosed diabetes among adults aged 20 to 79 years, United States, 1980–2012. JAMA : the journal of the American Medical Association. 2014;312(12):1218–1226. [DOI] [PubMed] [Google Scholar]
- 2.Beckles GL, Chou CF. Disparities in the Prevalence of Diagnosed Diabetes - United States, 1999–2002 and 2011–2014. MMWR Morbidity and mortality weekly report. 2016;65(45):1265–1269. [DOI] [PubMed] [Google Scholar]
- 3.Lawrence JM, Mayer-Davis EJ, Reynolds K, et al. Diabetes in Hispanic American youth: prevalence, incidence, demographics, and clinical characteristics: the SEARCH for Diabetes in Youth Study. Diabetes care. 2009;32 Suppl 2:S123–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Diabetes in LAC Adults. LAC Dept. of Public Health;2007. [Google Scholar]
- 5.Menchine M, Marzec K, Solomon T, Arora S. Fragile health status of Latino patients with diabetes seen in the emergency department of an urban, safety-net hospital. Ethnicity & disease. 2013;23(1):49–55. [PubMed] [Google Scholar]
- 6.Tang TS, Ayala GX, Cherrington A, Rana G. A Review of Volunteer-Based Peer Support Interventions in Diabetes. Diabetes Spectrum. 2011;24(2):85–98. [Google Scholar]
- 7.Tang TS, Funnell MM, Sinco B, Spencer MS, Heisler M. Peer-Led, Empowerment-Based Approach to Self-Management Efforts in Diabetes (PLEASED): A Randomized Controlled Trial in an African American Community. Annals of family medicine. 2015;13 Suppl 1:S27–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.van Dam HA, van der Horst FG, Knoops L, Ryckman RM, Crebolder HF, van den Borne BH. Social support in diabetes: a systematic review of controlled intervention studies. Patient education and counseling. 2005;59(1):1–12. [DOI] [PubMed] [Google Scholar]
- 9.Hu J, Wallace DC, McCoy TP, Amirehsani KA. A family-based diabetes intervention for Hispanic adults and their family members. The Diabetes educator. 2014;40(1):48–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Spencer MS, Kieffer EC, Sinco B, et al. Outcomes at 18 Months From a Community Health Worker and Peer Leader Diabetes Self-Management Program for Latino Adults. Diabetes care. 2018;41(7):1414–1422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Two Feathers J, Kieffer EC, Palmisano G, et al. Racial and Ethnic Approaches to Community Health (REACH) Detroit partnership: improving diabetes-related outcomes among African American and Latino adults. American journal of public health. 2005;95(9):1552–1560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Teufel-Shone NI, Drummond R, Rawiel U. Developing and adapting a family-based diabetes program at the U.S.-Mexico border. Preventing chronic disease. 2005;2(1):A20. [PMC free article] [PubMed] [Google Scholar]
- 13.Thompson JR, Horton C, Flores C. Advancing diabetes self-management in the Mexican American population: a community health worker model in a primary care setting. The Diabetes educator. 2007;33 Suppl 6:159s–165s. [DOI] [PubMed] [Google Scholar]
- 14.Bell AM, Fonda SJ, Walker MS, Schmidt V, Vigersky RA. Mobile phone-based video messages for diabetes self-care support. Journal of diabetes science and technology. 2012;6(2):310–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Seto E, Leonard KJ, Cafazzo JA, Barnsley J, Masino C, Ross HJ. Mobile phone-based telemonitoring for heart failure management: a randomized controlled trial. Journal of medical Internet research. 2012;14(1):e31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Quinn CC, Clough SS, Minor JM, Lender D, Okafor MC, Gruber-Baldini A. WellDoc™ mobile diabetes management randomized controlled trial: change in clinical and behavioral outcomes and patient and physician satisfaction. Diabetes technology & therapeutics. 2008;10(3):160–168. [DOI] [PubMed] [Google Scholar]
- 17.Quinn CC, Shardell MD, Terrin ML, Barr EA, Ballew SH, Gruber-Baldini AL. Cluster-randomized trial of a mobile phone personalized behavioral intervention for blood glucose control. Diabetes care. 2011;34(9):1934–1942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Arora S, Abramson T, Ruiz R, Coyne C, DeSantos R, Menchine M. Describing the Mobile Health Capacity of Inner City Latino Emergency Department Patients: Are National Estimates Accurate? Academic Emergency Medicine. 2013;20(S):187. [Google Scholar]
- 19.Livingston G. Latinos and digital technology, 2010. Pew Hispanic Center, web page, February. 2011;9. [Google Scholar]
- 20.Arora S, Ford K, Terp S, et al. Describing the evolution of mobile technology usage for Latino patients and comparing findings to national mHealth estimates. J Am Med Inform Assoc. 2016;23(5):979–983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Arora S, Peters AL, Burner E, Lam CN, Menchine M. Trial to examine text message-based mHealth in emergency department patients with diabetes (TExT-MED): a randomized controlled trial. Annals of emergency medicine. 2014;63(6):745–754.e746. [DOI] [PubMed] [Google Scholar]
- 22.Hay JW, Lee PJ, Jin H, et al. Cost-Effectiveness of a Technology-Facilitated Depression Care Management Adoption Model in Safety-Net Primary Care Patients with Type 2 Diabetes. Value Health. 2018;21(5):561–568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Burner E, Lam CN, DeRoss R, Kagawa-Singer M, Menchine M, Arora S. Using Mobile Health to Improve Social Support for Low-Income Latino Patients with Diabetes: A Mixed-Methods Analysis of the Feasibility Trial of TExT-MED + FANS. Diabetes technology & therapeutics. 2018;20(1):39–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Boudreaux ED, Bock B, O’Hea E. When an event sparks behavior change: an introduction to the sentinel event method of dynamic model building and its application to emergency medicine. Academic emergency medicine : official journal of the Society for Academic Emergency Medicine. 2012;19(3):329–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Galarraga JE, Mutter R, Pines JM. Costs associated with ambulatory care sensitive conditions across hospital-based settings. Academic emergency medicine : official journal of the Society for Academic Emergency Medicine. 2015;22(2):172–181. [DOI] [PubMed] [Google Scholar]
- 26.Diseases NIoDaDaK. About NDEP. http://ndep.nih.gov/about-ndep/index.aspx. Accessed September 12, 2012.
- 27.Arora S, Marzec K, Gates C, Menchine M. Diabetes knowledge in predominantly Latino patients and family caregivers in an urban emergency department. Ethnicity & disease. 2011;21(1):1–6. [PubMed] [Google Scholar]
- 28.Langford CP, Bowsher J, Maloney JP, Lillis PP. Social support: a conceptual analysis. Journal of advanced nursing. 1997;25(1):95–100. [DOI] [PubMed] [Google Scholar]
- 29.Burner ER, Menchine MD, Kubicek K, Robles M, Arora S. Perceptions of successful cues to action and opportunities to augment behavioral triggers in diabetes self-management: qualitative analysis of a mobile intervention for low-income Latinos with diabetes. Journal of medical Internet research. 2014;16(1):e25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)--a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42(2):377–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Toobert DJ, Hampson SE, Glasgow, RE. The summary of diabetes self-care activities measure: results from 7 studies and a revised scale. Diabetes care. 2000;23(7):943–950. [DOI] [PubMed] [Google Scholar]
- 32.Wilson IB, Fowler FJ Jr., Cosenza CA, et al. Cognitive and field testing of a new set of medication adherence self-report items for HIV care. AIDS Behav. 2014;18(12):2349–2358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Anderson RM, Fitzgerald JT, Gruppen LD, Funnell MM, Oh MS. The Diabetes Empowerment Scale-Short Form (DES-SF). Diabetes care. 2003;26(5):1641–1642. [DOI] [PubMed] [Google Scholar]
- 34.Polonsky WH, Fisher L, Earles J, et al. Assessing psychosocial distress in diabetes: development of the diabetes distress scale. Diabetes care. 2005;28(3):626–631. [DOI] [PubMed] [Google Scholar]
- 35.Kroenke K, Spitzer RL, Williams JB, Lowe B. The Patient Health Questionnaire Somatic, Anxiety, and Depressive Symptom Scales: a systematic review. Gen Hosp Psychiatry. 2010;32(4):345–359. [DOI] [PubMed] [Google Scholar]
- 36.Egede LE, Ellis C. Development and psychometric properties of the 12-item diabetes fatalism scale. Journal of general internal medicine. 2010;25(1):61–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Topp CW, Ostergaard SD, Sondergaard S, Bech P. The WHO-5 Well-Being Index: a systematic review of the literature. Psychother Psychosom. 2015;84(3):167–176. [DOI] [PubMed] [Google Scholar]
- 38.Chew LD, Griffin JM, Partin MR, et al. Validation of screening questions for limited health literacy in a large VA outpatient population. Journal of general internal medicine. 2008;23(5):561–566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lewin AB, Geffken GR, Heidgerken AD, et al. The diabetes family behavior checklist: A psychometric evaluation. Journal of Clinical Psychology in Medical Settings. 2005;12(4):315–322. [Google Scholar]
- 40.Fitzgerald JT, Davis WK, Connell CM, Hess GE, Funnell MM, Hiss RG. Development and validation of the Diabetes Care Profile. Eval Health Prof. 1996;19(2):208–230. [DOI] [PubMed] [Google Scholar]
- 41.Norbeck JS, Lindsey AM, Carrieri VL. The development of an instrument to measure social support. Nursing research. 1981;30(5):264–269. [PubMed] [Google Scholar]
- 42.Polonsky WH, Fisher L, Hessler D, Johnson N. Emotional Distress in the Partners of Type 1 Diabetes Adults: Worries About Hypoglycemia and Other Key Concerns. Diabetes technology & therapeutics. 2016;18(5):292–297. [DOI] [PubMed] [Google Scholar]
- 43.Bentler PM, Chou C-P. Practical issues in structural modeling. Sociological Methods & Research. 1987;16(1):78–117. [Google Scholar]
- 44.Chou C-P, Bentler PM. Estimates and tests in structural equation modeling. 1995.
- 45.Charmaz K Constructing grounded theory: A practical guide through qualitative analysis. Sage Publications Limited; 2006. [Google Scholar]
- 46.Dedoose [computer program]. Version 4.5.91. Los Angeles, CA: SocioCultural Resources Counsultants, LLC; 2012. [Google Scholar]
- 47.Data Survey. In: Services CUDoHaH, ed.
- 48.Rotheram-Borus MJ, Tomlinson M, Gwegwe M, Comulada WS, Kaufman N, Keim M. Diabetes buddies peer support through a mobile phone buddy system. The Diabetes educator. 2012;38(3):357–365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sylvetsky AC, Nandagopal R, Nguyen TT, et al. Buddy Study: Partners for better health in adolescents with type 2 diabetes. World journal of diabetes. 2015;6(18):1355–1362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Aikens JE, Trivedi R, Aron DC, Piette JD. Integrating support persons into diabetes telemonitoring to improve self-management and medication adherence. Journal of general internal medicine. 2015;30(3):319–326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mayberry LS, Berg CA, Harper KJ, Osborn CY. The Design, Usability, and Feasibility of a Family-Focused Diabetes Self-Care Support mHealth Intervention for Diverse, Low-Income Adults with Type 2 Diabetes. Journal of diabetes research. 2016;2016:7586385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Campbell DJ, Ronksley PE, Hemmelgarn BR, et al. Association of enrolment in primary care networks with diabetes care and outcomes among First Nations and low-income Albertans. Open Med. 2012;6(4):e155–165. [PMC free article] [PubMed] [Google Scholar]
- 53.Birtwhistle R, Green ME, Frymire E, et al. Hospital admission rates and emergency department use in relation to glycated hemoglobin in people with diabetes mellitus: a linkage study using electronic medical record and administrative data in Ontario. CMAJ Open. 2017;5(3):E557–E564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Cheung PT, Wiler JL, Lowe RA, Ginde AA. National study of barriers to timely primary care and emergency department utilization among Medicaid beneficiaries. Annals of emergency medicine. 2012;60(1):4–10 e12. [DOI] [PubMed] [Google Scholar]
- 55.Menchine MD, Arora S, Camargo CA, Ginde AA. Prevalence of undiagnosed and suboptimally controlled diabetes by point-of-care HbA1C in unselected emergency department patients. Academic emergency medicine : official journal of the Society for Academic Emergency Medicine. 2011;18(3):326–329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hu J, Amirehsani K, Wallace DC, Letvak S. Perceptions of barriers in managing diabetes: perspectives of Hispanic immigrant patients and family members. The Diabetes educator. 2013;39(4):494–503. [DOI] [PMC free article] [PubMed] [Google Scholar]