Figure 1. A) Oxidized lipids/cholesterol are risk factors for clinically significant thrombotic events in cardiovascular disease.
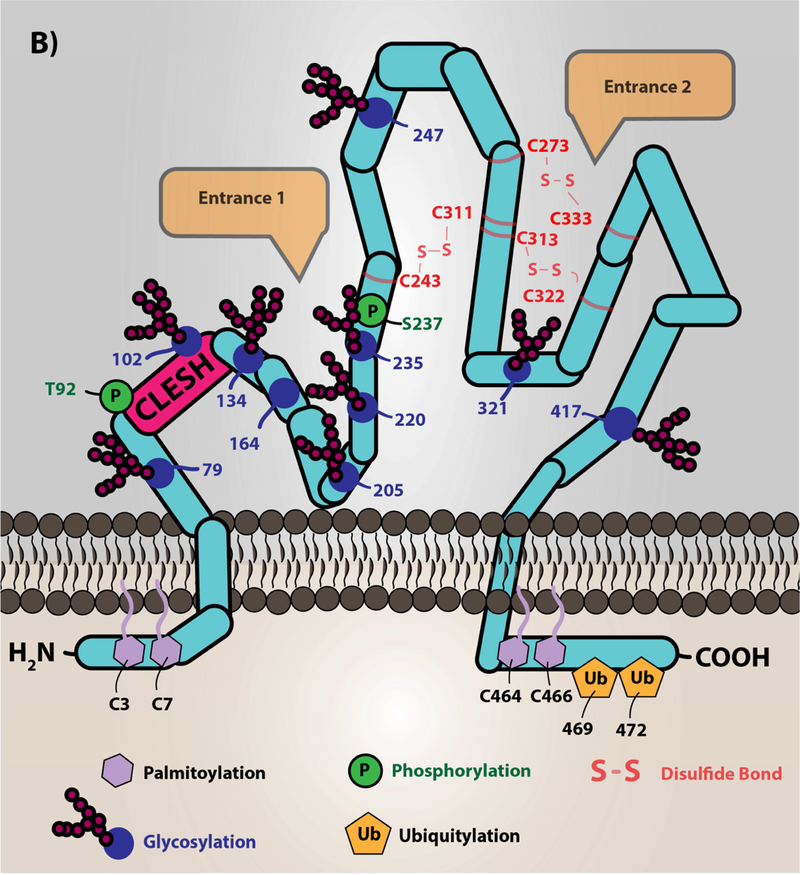
Low density lipoprotein particles, which are cholesterol carrying molecules, become oxidized during the inflammatory and oxidative processes of atherosclerotic plaque formation. The major targets of redox active species are the protein, cholesterol, and lipid components of the particle. Phospholipid oxidation at the sn-2 position by these species promotes truncation of the lipid and subsequent incorporation of oxygen. The specific oxidized lipid motif, known as oxPCCD36, is shown, which is predominantly oxidized phosphatidylcholine species with a carboxylic acid or aldehyde in the terminal position, a double bond in the beta carbon, and a ketone or alcohol in the gamma position. These oxidized lipids translocate to the aqueous plasma milieu forming so-called lipid “whiskers” that are recognized selectively by scavenger receptor CD36 present abundantly on platelets. Cholesterol oxidation yields keto- and alcohol-derivatives of the cholesterol. Modification of the protein component of the lipoprotein is amino acid-dependent (e.g. tyrosine nitration, aldehyde adduction on lysines). B.) Scavenger receptor CD36 topology and structure. The depiction of CD36 on the membrane is shown with various post-translational modifications. CD36 is palmitoylated on the two short N- and C-terminal tails with ubiquitination sites in the C-terminal tail. CD36 has two membrane-spanning domains that are involved in protein-protein interaction with itself to form dimers and with other receptors clustered in membrane lipid microdomains, such as toll-like receptors (55,104), sodium-potassium ATPase (57), tetraspanin CD9 (54), and others (11). The extracellular domain of CD36 is heavily glycosylated, which functions to traffic the protein to the correct membrane compartment. Residues 92–120 are known as the CLESH domain, and are essential for binding to the type 1 thrombospondin repeat domain (TSR) found in anti-angiogenic proteins of the thrombospondin family. Furthermore, a hydrophobic pocket represents a potential domain for fatty acid and oxidized lipid interaction. CD36 has two potential phosphorylation sites on the extracellular domain (Thr92 and Ser237). Three disulfide bonds within the extracellular domain are essential to maintain the structure of the protein. Entrance 1 in the figure represents a hydrophobic pocket for interaction with various ligands. Entrance 2 in the figure represents a hypothesized channel for fatty acid transportation as suggested by structural modeling with the LIMP-II member of the scavenger receptor family. The mouse anti-CD36 monoclonal antibody [clone FA6–152], which is predominantly used as an inhibitor, was shown to bind to amino acids 155–183 and inhibits the ligand binding capacity. However, the detailed mechanism of inhibiting CD36 signal transduction by this antibody is not clear and is potentially due to masking the receptor-ligand binding site or changing the conformation of the protein.
Image (B) was originally printed in Yang, X. et al. (2017) Nature Review It was modified, and reprinted with permission from Springer Nature