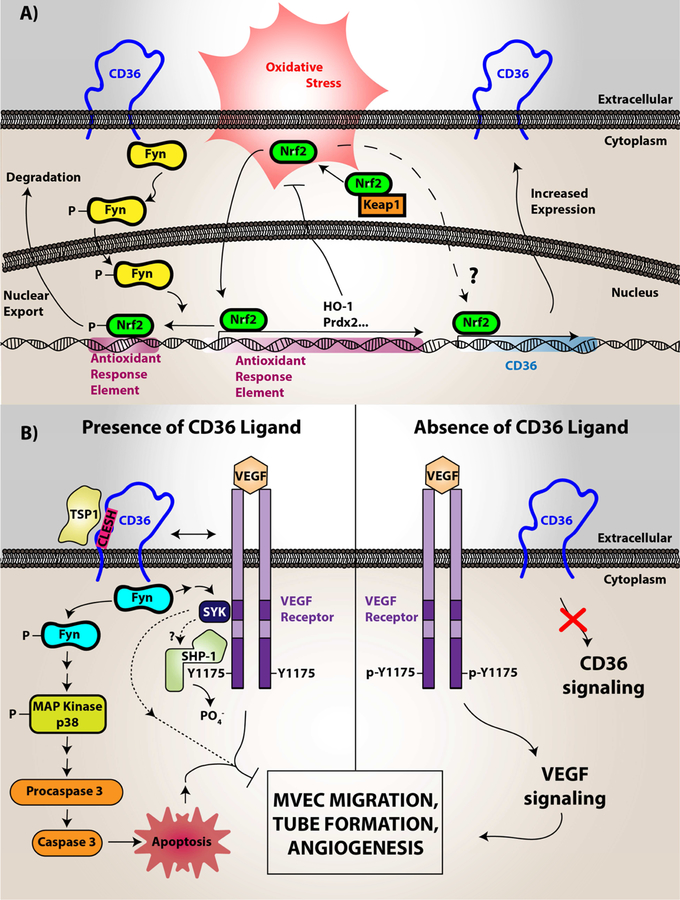
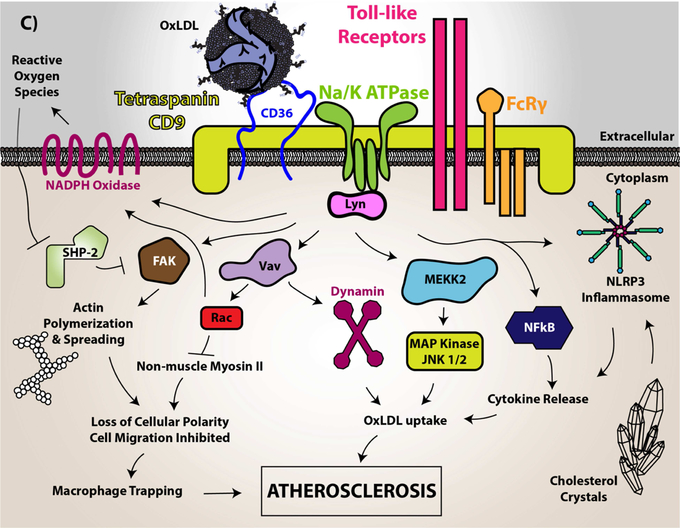
Figure 2. CD36 redox signaling in vascular smooth muscle cells, microvascular endothelial cells, and monocytes/macrophages.
(A) In vascular smooth muscle cells, CD36 signaling was shown to downregulate the antioxidant transcription factor Nrf2. Nrf2 is normally a cytosolic protein held in an inactive state by binding to an inhibitory partner known as KEAP or Inhibitor of Nrf2 (INrf2). Upon oxidative stress the two proteins dissociate and Nrf2 translocates to the nucleus where it binds to specific DNA sequences known as antioxidant response elements (ARE) to promote transcription of multiple antioxidant genes, including peroxiredoxin 2 (Prdx2) and heme oxygenase-1 (HO-1). CD36 signaling via the Src family kinase Fyn, leads to phosphorylation of nuclear Nrf2, nuclear export, and degradation, thereby preventing upregulation of antioxidants and thus promoting reactive oxygen species accumulation and pro-atherogenic cellular activity. (B) In microvascular endothelial cells (MVEC), vascular endothelial growth factor receptor 2 (VEGFR2) recognizes its ligand VEGF and becomes phosphorylated. VEGFR2 than transduces signals to mediate cellular migration, proliferation, and tube formation important for angiogenesis. However, CD36 through recognition of thrombospondin-1 and related proteins promotes activation of Fyn, p38 MAP kinase, and apoptosis initiating caspases, which overall limits endothelial cell migration and tube formation. CD36 also forms functional complexes with VEGFR2, promoting a spleen tyrosine kinase (Syk)-dependent phosphorylation and activation of the Src-homology Protein Tyrosine Phosphatase 1 (SHP-1), which dephosphorylates VEGFR2 to limit MVEC function. (C) In macrophages, CD36 was shown to form a complex with multiple proteins in membrane microdomains, including CD9, Toll-Like Receptors, Fc Receptor γ, and Na-K ATPase. Upon oxLDL binding, CD36 promotes direct recruitment and activation of Src family kinases (particularly Lyn via its association with Na-K ATPase) for signal transduction. This signal transduction promotes the phosphorylation of focal adhesion kinases for cytoskeletal rearrangement, loss of cellular locomotion, and macrophage trapping. In addition, this signal transduction increases the generation of reactive oxygen species from NADPH oxidase. These species modify the protein tyrosine phosphatase SHP2 inactivating the enzyme, which regulates Src kinase-mediated signaling. oxLDL recognition by CD36 also promotes Vav family guanine nucleotide exchange factors that promote Rac activity to modulate non-muscle myosin II and regulation of cellular locomotion. Vavs also connect CD36 to an oxLDL-uptake mechanism that is dependent on dynamin. In relation to oxLDL uptake, MAP kinase activation (JNK1/2) was shown to be essential for CD36-mediated macrophage foam cell formation. The mechanistic connection between CD36, Toll-like receptors, and Na-K ATPase were shown to be linked to activation of pro-inflammatory pathways, including the NF-kB pathway and activation of the NLRP3 inflammasome, that promotes atherosclerosis. The CD36 and Toll-like receptor signaling pathway was linked to cholesterol crystal deposition and foam cell formation.
Image (A) was originally printed in Li W. et al. (2010) Journal of Clinical Investigations by the American Society of Clinical Investigations. It was modified and reprinted with permission.
Image (B) was originally published in Chu L.Y. et al. (2013) Blood by the American Society of Hematology. It was modified and reprinted with permission.
Image (C) image was originally printed in Park Y.M. (2014) Experimental and Molecular Medicine by Springer Nature. It was modified and reprinted.