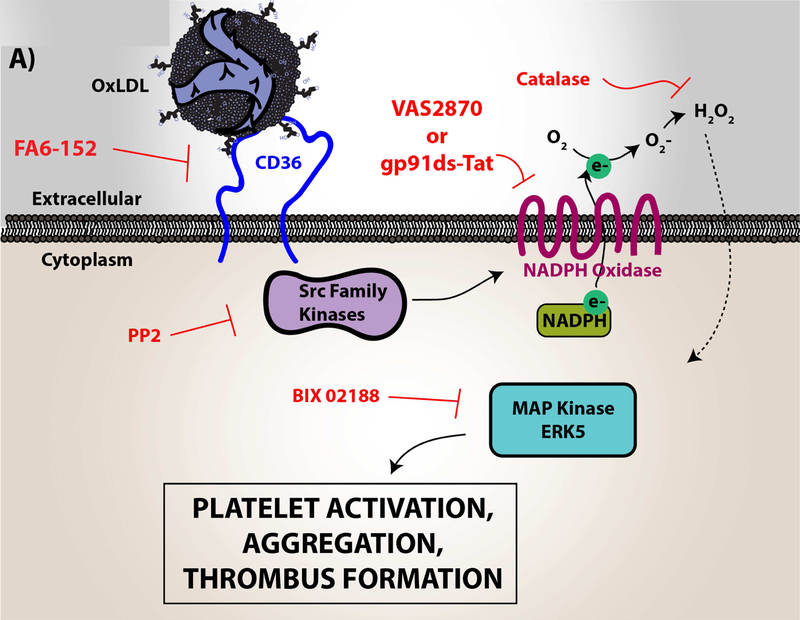
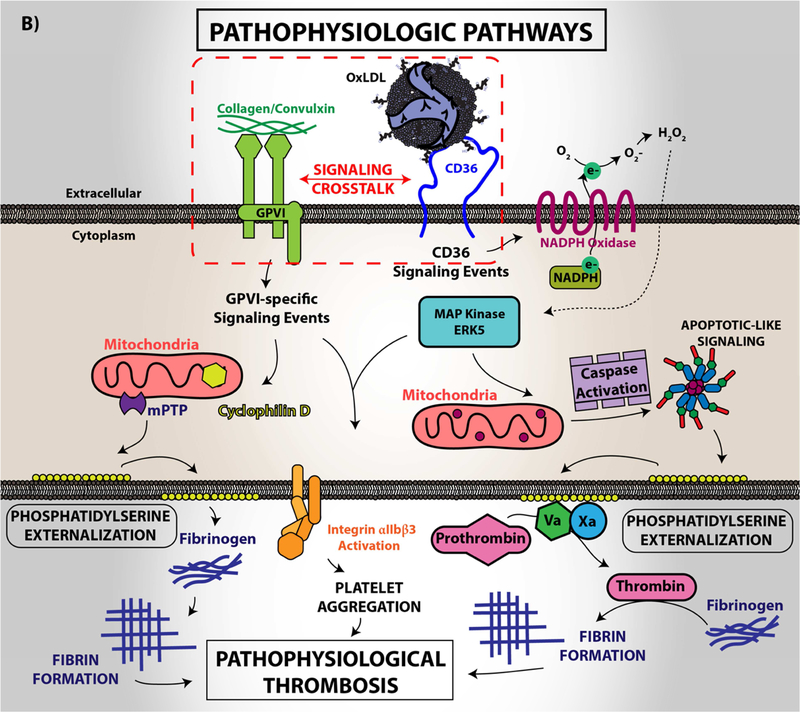
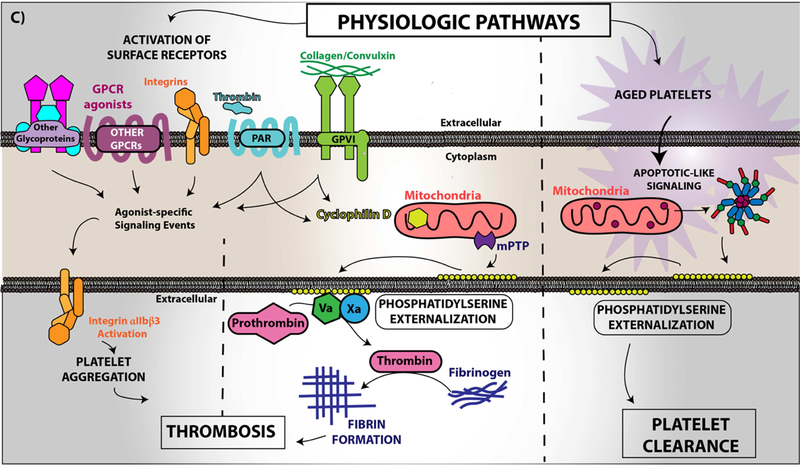
Figure 4. Platelet CD36 redox signaling promotes phosphatidylserine externalization.
CD36 signal transduction in platelets is via a redox pathway that promotes pathophysiologic thrombosis. In (A), CD36 signaling activates Src family kinases, predominantly Fyn and Lyn, that promotes downstream generation of specific reactive oxygen species, superoxide radical anion and hydrogen peroxide, from NADPH oxidase. Superoxide radical and hydrogen peroxide then activate redox-sensitive signaling pathways, including the MAP kinase ERK5. ERK5 links platelet CD36 to a prothrombotic phenotype. Interruption of this pathway by either pharmacologically, by blocking antibodies, or through genetic deficiency of key components prevented platelet activation and subsequent thrombosis in dyslipidemia. In (B), under pathophysiologic conditions, redox-dependent events initiated by CD36 enhances phosphatidylserine externalization predominantly through apoptotic-like caspase signaling. This ERK5 dependent pathway cross-talks with the collagen receptor GPVI pathway to enhance phosphatidylserine exposure, subsequent fibrin formation, and promote pathological arterial thrombosis. In (C), the physiologic pathways of phosphatidylserine externalization and integrin activation are described to the left, where physiologic agonists activate their receptors and triggers receptor-dependent signaling pathways. In one phenotype, signaling by physiologic agonists promote activation of integrin aIIbb3 that leads to platelet aggregation and subsequent thrombosis. In another phenotype, potent physiologic agonists activate the peptidylprolyl isomerase cyclophilin D to sensitize the formation of the mitochondrial permeability transition pore that enhances procoagulant phosphatidylserine externalization. This mechanism promotes assembly of the prothrombinase complex to promote thrombin generation and subsequent fibrin deposition to support thrombosis. An alternative physiologic pathway to phosphatidylserine externalization was described, in which aged platelets promote phosphatidylserine externalization through apoptotic-like signaling. Phosphatidylserine exposure in this setting enhances platelet clearance.