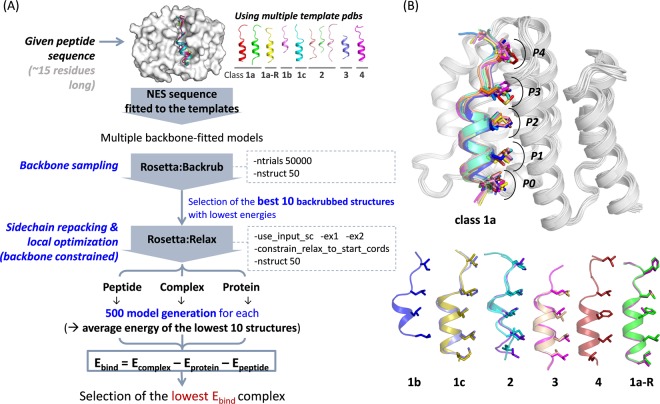
Figure 5.
Structure-based prediction of the stability of CRM1-NES peptide complex. (A) CRM1-NES peptide complex model generation and Ebind calculation procedure. (B) Generated models for the complex structures of CRM1-NES peptides with lowest Ebind. Class 1a peptides are displayed with CRM1 (in white) at the top. The hydrophobic (Φ) residues of these NES peptides (shown in the sticks) occupy the corresponding hydrophobic pockets (P0-P4) in CRM1. Peptides of other classes are shown at the bottom with the hydrophobic residues shown in the sticks.