Abstract
Chloroform, ethyl acetate and methanol extracts from the aerial parts of Ferula caspica M. Bieb. were tested for their antioxidant capacities by CUPRAC, ABTS, FRAP, Folin–Ciocalteu and aluminum chloride methods and for antimicrobial activities by the broth microdilution method. Chloroform and ethyl acetate extracts showed the highest antioxidant capacity and antimicrobial activity. Three known sesquiterpene derivatives; 1-(2′,4′-dihydroxyphenyl)-3,7,11-trimethyl-3-vinyl-6(E),10-dodecadien-1-one (1), 2,3-dihydro-7-hydroxy-2,3-dimethyl-2-[4′,8′-dimethyl-3′,7′-nonadienyl]-furo[3,2,c]coumarin (2), 2,3-dihydro-7-hydroxy-2,3-dimethyl-3-[4′,8′-dimethyl-3′,7′-nonadienyl]-furo[3,2,c]coumarin(3); phenylpropanoid; laserine/2-epilaserine (4/5) and steroid mixtures; stigmasterol and β-sitosterol (6/7) were isolated from chloroform extract; three known flavonoids; kaempferol-3-O-β-glucopyranoside (8), kaempferol-3-O-α-rhamnopyranoside (9), quercetin-3-O-β-glucopyranoside (10), and one benzoic acid derivative; 2,4-dihydroxybenzoic acid (11) were isolated from the ethyl acetate extract. The structures were elucidated by spectroscopic methods.
Keywords: Ferula caspica, Constituents, NMR, Antimicrobial, Antioxidant
1. Introduction
The genus Ferula L. distributed worldwide (Pimenov and Leonev, 2004) mainly in central and South-west Asia and Mediterranean basin (Yaqoob and Nawchoo, 2016) is the third largest genus of Apiaceae (Pimenov and Leonev, 2004). In Turkey, the genus Ferula has 23 species, 13 of which are endemic to Turkish Flora. (Peşmen, 1972, Duman and Sagiroglu, 2005, Sağıroğlu, 2005, Sagiroglu and Duman, 2007, Sagiroglu and Duman, 2010, Pimenov and Kljuykov, 2013, Sağıroğlu and Duman, 2014). Ferula species are traditionally used in the treatment of various diseases. Aerial parts of F. rigidula DC. subsp. rigidula, all parts of F. orientalis L., the roots of F. elaeochytris Korovin and F. longipedunculata Peşmen are used as immunostimulant, aphrodisiac, antidiabetic, anticholesterolemic, emmenagogue, menstrual regulator and for treatment of gastric pain in Anatolia (Altundag and Ozturk, 2011, Güneş and Özhatay, 2011, Demirci and Özhatay, 2012, Güzel et al., 2015, Mükemre et al., 2015). Phytochemical studies on Ferula species showed the presence of daucane, germacrane, humulane type sesquiterpenes (Miski and Mabry, 1986, Miski et al., 1987, Miski and Jakupovic, 1990, Ahmed, 1991, Galal et al., 2001, Lhuillier et al., 2005, Alkhatib et al., 2010), sesquiterpene lactone derivatives (Iranshahi et al., 2008, Kurimoto et al., 2012) and sesquiterpene coumarins (Miski et al., 1985, Yang et al., 2006, Abd et al., 2007, Iranshahi et al., 2009, Iranshahi et al., 2010, Dastan et al., 2014, Li et al., 2015a, Li et al., 2015b). Furthermore, furanocoumarin, asetophenone, benzofuran sesquiterpenes (Kojima et al., 1998, Chen et al., 2001, Meng et al., 2013a, Meng et al., 2013b) and flavonoids (Znati et al., 2014) were isolated from these species. In this study, we aimed to investigate the antioxidant and antimicrobial activities of different extracts from Ferula caspica M. Bieb. and to isolate polar and apolar secondary metabolites from the active extracts.
2. Material and methods
2.1. General experimental procedures
NMR spectra (600 MHz for 1H NMR and 125 MHz for 13C NMR) were measured on Agilent spectrometry. HR-ESI-MS spectra were recorded on the Bruker micro Q-TOF/6500 mass spectrometer. Silica gel 60 (0.063–0.200 mm/70–230 mesh, Merck, Germany), Sephadex LH 20 (Merck, Germany) and reversed-phase material (C-18, LiChroprep 25–40 µm, Merck, Germany) were used for column chromatography (CC) and Silica gel 60 F254 (Merck, Germany) was used for Thin Layer Chromatography (TLC) and Preparative Thin Layer Chromatography (pTLC). Detection of spots on the plates was done with 1% Vanillin-sulphuric acid or ceric sulfate reagents and UV light (Camag 254 and 366 nm). Mueller-Hinton Broth (MHB, BBL, MD, USA), RPMI-1640 medium (ICN-Flow, Aurora, OH-USA) with L-glutamine and 3-(N-morpholino)propane sulphonic acid (MOPS) (Buffer-ICN-Flow, Aurora, OH-USA) were used for the antimicrobial activity. Reagents and standard compounds used in antioxidant capacity assays were purchased from Sigma-Aldrich (USA).
2.2. Plant material and the extraction procedure
Plant materials were collected from Nallıhan-Davutoğlan Bird Paradise, Ankara province Central Anatolia Region of Turkey in June 2011. The specimens were identified by Prof. Dr. Hayri Duman, Gazi University, Faculty of Science, Department of Botany, Ankara, Turkey. Voucher specimens were deposited at the Herbarium of the Faculty of Pharmacy, Hacettepe University, Ankara (HUEF 11003).
Air dried and powdered aerial parts of F. caspica (597 g) were extracted with chloroform (5 L × 4) to give 27 g extract (yield: 4.5%) and methanol (5 L × 4) to give 64 g extract (yield: 10.7%), respectively, using rotary extractor under 40 °C without vacuum. The methanol extract was partitioned between ethyl acetate and water to yield 5 g EtOAc extract (yield: 0.8%) (see Fig. 1).
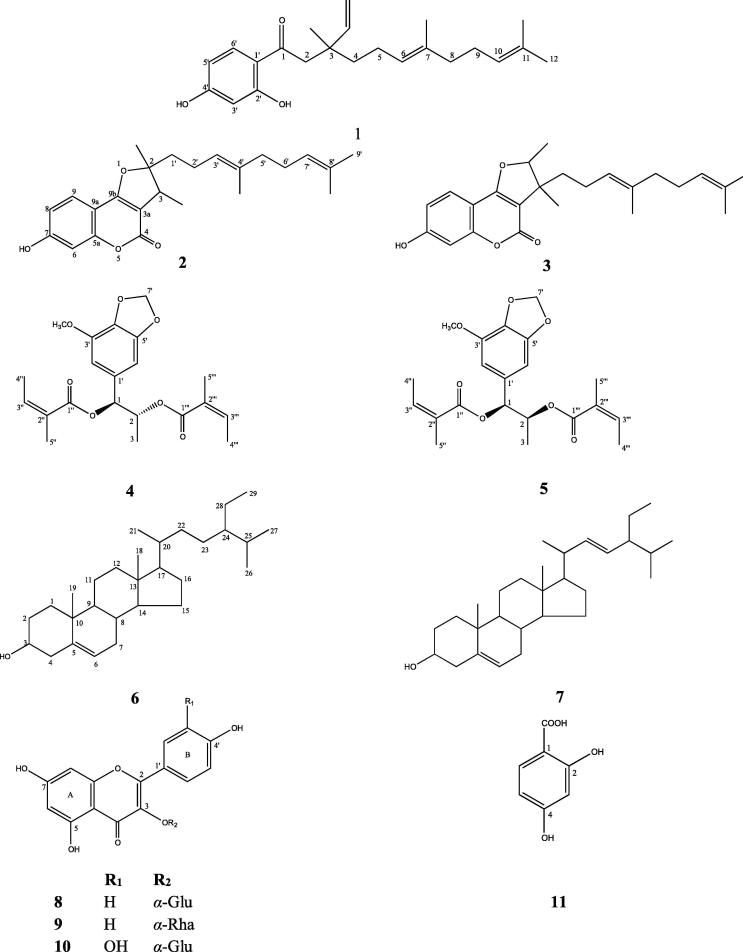
Fig. 1.
Structures of compounds 1–11.
2.3. Isolation of the compounds 1–11
Chloroform extract (27 g) was fractionated by silica gel column chromatography using n-hexane and EtOAc with increasing polarity (95:5–0:100) to give 7 fractions (Frs. A–G). Fr. B (577 mg) was refractionated by silica gel column chromatography (CC) using n-hexane and acetone (95:5–90:10) to yield 5 fractions (Frs. B1–5). Then, Fr. B1 (30 mg) subjected to pTLC (n-hexane-acetone, 3:2) to afford the mixture of 4/5 (10 mg). In a sequential manner, Fr. B4 (150 mg) was chromatographed on silica gel eluting by increasing polarity of acetone in n-hexane (90:10–0:100), sephadex LH-20 (cyclohexane-CH2Cl2-EtOH, 7:4:1) and pTLC (n-Hekzane-EtOAc, 3:2) to purify compound 1 (26 mg). Fr. D (300 mg) was submitted to silica gel CC eluted with n-hexane-EtOAc (9:1) and then the mixture of compounds 6/7 was yielded from Fr. D2 (77.5 mg) with pTLC (toluen-EtOAc-acetonitrile, 40:9:1). Fr. F (1 g) was resubmitted to silica gel CC with the increasing volume of EtOAc in toluene (80:20–0:100). Fr. F2 (145.5 mg) was subjected to silica gel (toluen-EtOAc, 97:3–92.5:7.5), Sephadex LH-20 CC (cyclohexane-CH2Cl2-EtOH, 7:4:1) and pTLC (toluen-EtOAc-acetonitrile, 40:9:1) to obtain compound 3 (10 mg). Compound 2 (6 mg) was purified from Fr. F4 (147.8 mg) with silica gel CC (toluen-EtOAc, 96:4–91:9) and pTLC (toluen-EtOAc-acetonitrile, 40:9:1).
EtOAc extract (5.0 g) was fractionated by Reverse Phase-Vacuum Liquid Chromatography (RP-VLC) (H2O-MeOH, 100:0–0:100) to give fractions (Frs. 1–6). Fr. 2 (200 mg) refractionated by RP-VLC (H2O-MeOH, 100:0–0:100) to yield sequential fractions and then compound 11 (10 mg) was gained from Fr. 2b (40 mg) by pTLC (CHCl3-MeOH-H2O, 61:32:7). Compound 10 (10 mg) and 8 (7 mg) were purified from Frs. 3 (100 mg) and 5 (100 mg), respectively by applying silica gel CC (CHCl3-MeOH, 90:10–0:100 for Fr. 3 and EtOAc-MeOH-H2O, 100:5:1 for Fr. 5) and pTLC (CHCl3-MeOH-H2O, 61:32:7 for Fr. 3 and EtOAc-MeOH-H2O, 100:13.5:10 for Fr. 5). And finally, Fr. 6 (86.5 mg) was chromatographed on silica gel (CHCl3-MeOH, 98:2–87:13) to give sequential fractions and Fr 6b (27 mg) was applied on Sephadex LH-20 CC (MeOH) to yield compound 9 (13.2).
2.4. Antimicrobial and antioxidant activity assays
The extracts were tested against the bacterial (Escherichia coli ATCC 25922, Enterococcus faecalis ATCC 29212, Pseudomonas aeruginosa ATCC 27853, Staphylococcus aureus ATCC 29213) and fungal species (Candida albicans ATCC 90028, C. krusei ATCC 6258, C. parapsilosis ATCC 90018) by using broth microdilution method reported by the Clinical and Laboratory Standards Institute (Wayne, 2008a, Wayne, 2008b). Gentamycin and fluconazole were used as reference compounds. The test was performed in Mueller-Hinton Broth (MHB, BBL, MD, USA) for bacteria. RPMI-1640 medium (ICN-Flow, Aurora, OH-USA) with L-glutamine, buffered with 3-(N-morpholino)propane sulphonic acid (MOPS) (Buffer-ICN-Flow, Aurora, OH-USA) at pH = 7.4 was used as the test medium for fungi. The microtiter plates were incubated at 35 °C for 18–24 h for bacteria and 48 h for fungi. Minimum inhibitory concentrations (MIC) were defined visually as the lowest concentrations of the extracts that had no turbidity.
Antioxidant capacities of the obtained extracts were evaluated by the following methods:2,2′-azino-bis(3-ethylbenzothiazoline-6-sulphonic acid) (ABTS) (Re et al., 1999) radical cation scavenging activity, ferric-reducing antioxidant power (FRAP) (Oyaizu, 1986) and cupric ion reducing antioxidant capacity (CUPRAC) (Apak et al., 2004). The quantitative determinations of flavonoids and phenolic compounds of all extracts were conducted aluminum chloride method (Chang et al., 2002) and Folin–Ciocalteu assay (Slinkard and Singleton, 1977), respectively. Briefly, after adding 200 µL of diluted ABTS solution to 20 µL of different concentrations of the test samples in ABTS radical scavenging assay the absorbance were measured at 734 nm. In CUPRAC assay, 50 µL of each of copper(II) chloride, neocuproine, and ammonium acetate solutions were mixed with 25 µL of the test sample and 25 µL of water. After incubating 30 min, absorbance was measured at 450 nm. According to Oyaizu’s assay, after incubating at 50 °C for 20 min, the mixture of test sample (20 µL), sodium phosphate buffer (pH 6.6, 0.2 M, 50 µL) and potassium ferricyanide (50 µL, 1%, w/v) were acidified by adding trichloroacetic acid (50 µL, 10% w/v). 50 µL of this solution was mixed with distilled water (50 µL) and iron(III) chloride (10 µL, 0.1% w/v). Absorbance was read at 700 nm after 30 min incubation. The absorbance of the mixture of the test sample (25 µL), ethanol (75 µL, 95%), aluminum chloride (5 µL, 10%), potassium acetate (5 µL, 1 M) and distilled water (140 µL) was measured at 415 nm in aluminum chloride method. Finally, according to Folin-Ciocalteu assay of Slinkard and Singleton, the absorbance of the mixture of 100 µL of diluted Folin reagent, 20 µL of the test sample and 80 µL of sodium carbonate (7.5%) solution, after 2 h incubation, was measured at 765 nm.
2.5. Statistical analysis
Obtained data from antioxidant capacity assays were analyzed using one-way ANOVA followed by Tukey test, p-values < 0.05 were considered statistically significant.
3. Results
Isolation studies were performed on chloroform and ethyl acetate extracts showed the highest activity in the assays for antimicrobial and antioxidant activities, respectively.
3.1. Structural elucidation of compounds 1–11
The chloroform extract obtained from the aerial parts of F. caspica was fractionated by a serial chromatographic procedures with normal-phase silica gel, Sephadex LH-20 and preparative thin layer chromatography (pTLC) to afford three known sesquiterpene derivatives (1–3) as well as known phenylpropanoid (4/5) and steroid (6/7) mixtures; 1-(2′,4′-dihydroxyphenyl)-3,7,11-trimethyl-3-vinyl-6(E),10-dodecadien-1-one (1) (Kojima et al., 1998), 2,3-dihydro-7-hydroxy-2,3-dimethyl-2-[4′,8′-dimethyl-3′,7′-nonadienyl]-furo[3,2,c]coumarin (2) (Isaka et al., 2001), 2,3-dihydro-7-hydroxy-2,3-dimethyl-3-[4′,8′-dimethyl-3′,7′-nonadienyl]-furo[3,2,c]coumarin (3) (Kojima et al., 2000), laserine/2-epilaserine mixture (4/5) (Barrero et al., 1992), stigmasterol and β-sitosterol mixture (6/7) (Chaturvedula and Prakash, 2012).
The ethyl acetate fraction of methanolic extract of the dried aerial parts of F. caspica was subjected to column chromatography over reversed-phase RP-18 silica gel, normal-phase silica gel, Sephadex LH-20 and pTLC to give three known flavonoids (8–10) and a benzoic acid derivative (11); kaempferol-3-O-β-glucopyranoside (8) (Han et al., 2004), kaempferol-3-O-α-rhamnopyranoside (9) (Correia et al., 2008), quercetine-3-O-β-glucopyranoside (10) (Han et al., 2004) and 2,4-dihydroxybenzoic acid (11) (Scott, 1972).
Structures of the compounds were determined by comparison of their spectroscopic data with those reported in the literatures.
3.1.1. Spectral data of compounds 1–11
1-(2′,4′-dihydroxyphenyl)-3,7,11-trimethyl-3-vinyl-6(E),10-dodecadien-1-one (1): 1H NMR (600 MHz, CDCl3) of 1: δH 7.64 (1H, d, J = 8.2, H-6′), 6.37 (1H, d, J = 3, H-5′), 6.36 (1H, s, H-3′), 5.86 (1H, dd, J = 17.6/10.5 Hz, Vinyl-CH), 5.09 (1H, †, H-6), 5.08 (1H, †, H-10), 5.02 (1H, d, J = 11, Vinyl-CH2a), 4.96 (1H, d, J = 17.6 Hz, Vinyl-CH2b), 2.89 (2H, d, J = 4.1 Hz, H-2), 2.05 (2H, m, H-9), 1.95 (2H, †, H-5), 1.95 (2H, †, H-8), 1.68 (3H, s, H-12), 1.59 (3H, s, H-11Me), 1.58 (3H, s, H-7Me), 1.53 (2H, m, H-4), 1.17 (3H, s, H-3Me); 13C NMR (150 MHz, CDCl3): δC 204.1 (C-1), 165.5 (C-2′), 162.6 (C-4′), 145.7 (Vinyl-CH), 133.2 (C-6′), 135.1 (C-7), 131.3 (C-11), 124.3 (C-10), 124.2 (C-6), 115.1 (C-1′), 112.2 (Vinyl-CH2), 107.6 (C-5′), 103.5 (C-3′), 47.1 (C-2), 41.1 (C-4), 40.3 (C-3), 39.7 (C-8), 26.7 (C-9), 25.7 (C-12), 23.2 (C-3Me), 22.8 (C-5), 17.7 (C-11Me), 16 (C-7Me).
2,3-dihydro-7-hydroxy-2,3-dimethyl-2-[4′,8′-dimethyl-3′,7′-nonadienyl]-furo[3,2,c]coumarin (2): 1H NMR (600 MHz, CDCl3) of 2: δH 7.53 (1H, d, J = 8.6 Hz, H-9), 7.18 (1H, d, J = 2.4 Hz, H-6), 6.86 (1H, dd, J = 8.6/2.3 Hz, H-8), 5.1 (1H, t, J = 7.0 Hz, H-3′), 5.06 (1H, t, J = 7.0 Hz, H-7′), 3.29 (1H, q, J = 7.0 Hz, H-3), 2.12 (2H, m, H-2′), 2.04 (2H, m, H-6′), 1.95 (2H, m, H-5′), 1.8 (2H, m, H-1′), 1.67 (3H, s, H-9′), 1.58 (3H, s, H-4′Me), 1.58 (3H, s, H-8′Me), 1.46 (3H, s, H-2Me), 1.31 (3H, d, J = 7.0 Hz, H-3Me); 13C NMR (150 MHz, CDCl3): δC 166.1 (C-9b), 162.5 (C-4), 161.2 (C-7), 156.7 (C-5a), 135.9 (C-4′), 131.4 (C-8′), 124.1 (C-9), 124.1 (C-7′), 123.1 (C-3′), 113.4 (C-8), 105.5 (C-9a), 103.1 (C-3a), 103.1 (C-6), 97.1 (C-2), 41.8 (C-3), 41.7 (C-1′), 39.6 (C-5′), 26.6 (C-6′), 25.6 (C-9′), 22.1 (C-2′), 20.4 (C-2Me), 16 (C-4′Me), 16 (C-8′Me), 14.7 (C-3Me).
2,3-dihydro-7-hydroxy-2,3-dimethyl-3-[4′8′-dimethyl-3′,7′-nonadienyl]-furo[3,2,c]coumarin (3): 1H NMR (600 MHz, CDCl3) of 2: δH 7.54 (1H, dd, J = 8.6/1.9 Hz, H-9), 7.21 (1H, d, J = 2.2 Hz, H-6), 6.87 (1H, dd, J = 8.6/2.2 Hz, H-8), 5.03 (1H, t, J = 6.8 Hz, H-3′), 5.03 (1H, t, J = 6.8 Hz, H-7′), 4.64 (1H, q, J = 6.7 Hz, H-2), 1.99 (2H, †, H-6′), 1.91–2.06 (2H, †, H-2′), 1.88 (2H, †, H-5′), 1.65 (2H, †, H-1′), 1.64 (3H, s, H-9′), 1.56 (3H, s, H-8′Me), 1.54 (3H, d, J = 6.8 Hz, H-2Me), 1.48 (3H, s, H-4′Me), 1.45 (3H, s, H-3Me); 13C NMR (150 MHz, CDCl3): δC 167.4 (C-9b), 162.4 (C-4), 161.3 (C-7), 156.8 (C-5a), 135.3 (C-4′), 131.3 (C-8′), 124.3 (C-9), 124.2 (C-7′), 124.0 (C-3′), 113.7 (C-8), 105.7 (C-9a), 105.5 (C-3a), 103.2 (C-6), 93.4 (C-2), 46.6 (C-3), 34.8 (C-1′), 39.6 (C-5′), 26.6 (C-6′), 25.7 (C-9′), 23.8 (C-2′), 13.9 (C-2Me), 23.4 (C-3Me), 17.7 (C-8′Me), 16.0 (C-4′Me).
Laserine (4): 1H NMR (600 MHz, CDCl3) of 4: δH 6.58 (1H, d, J = 1.5 Hz, H-6′), 6.56 (1H, d, J = 0.9 Hz, H-2′), 6.09 (1H, m, H-3″), 6.05* (1H, m, H-3‴), 5.97* (2H, s, H-7′), 5.76 (1H, d, J = 7.4 Hz, H-1), 5.35 (1H, dq, J = 7.3/6.5 Hz, H-2), 3.87 (3H, s, OMe), 1.97 (3H, dq, J = 7.3/1.5 Hz, H-4″), 1.93* (3H, dq, J = 7.3/1.5 Hz, H-4‴), 1.88 (3H, p, J = 1.5 Hz, H-5″), 1.85* (3H, p, J = 1.5 Hz, H-5‴), 1.16 (3H, d, J = 6.5 Hz, H-3); 13C NMR (150 MHz, CDCl3): δC 167.1* (C-1‴), 166.6** (C-1″), 148.9 (C-5′), 143.5* (C-3′), 139.0 (C-3″), 138.2*(C-3‴), 135.4 (C-4′), 131.7* (C-1′), 127.8* (C-2‴), 127 (C-2″), 107.2 (C-2′), 101.6 (C-6′), 101.6 (C-7′), 77.1 (C-1), 71.2 (C-2), 56.6 (OMe), 20.6 (C-5″), 20.6* (C-5‴), 16.8 (C-3), 15.8 (C-4″), 15.7 (C-4‴).
2-Epilaserine (5): 1H NMR (600 MHz, CDCl3) of 5: δH 6.58 (1H, d, J = 1.5 Hz, H-6′), 6.56 (1H, d, J = 0.9 Hz, H-2′), 6.13 (1H, m, H-3″), 6.06* (1H, m, H-3‴), 5.96* (2H, s, H-7′), 5.91 (1H, d, J = 4 Hz, H-1), 5.28 (1H, dq, J = 6.5/4.5 Hz, H-2), 3.87 (3H, s, OMe), 2.01 (3H, dq, J = 7.3/1.5 Hz, H-4″), 1.95* (3H, dq, J = 7.5/1.5 Hz, H-4‴), 1.96 (3H, p, J = 1.5 Hz, H-5″), 1.84* (3H, p, J = 1.5 Hz, H-5‴), 1.26 (3H, d, J = 6.5 Hz, H-3); 13C NMR (150 MHz, CDCl3): δC 167.2* (C-1‴), 166.5* (C-1″), 149 (C-5′), 143.4** (C-3′), 139.3 (C-3″), 138.3* (C-3‴), 135 (C-4′), 131.6* (C-1′), 127.7* (C-2‴), 127 (C-2″), 106.9 (C-2′), 101.2 (C-6′), 101.6 (C-7′), 75.9 (C-1), 71.6 (C-2), 56.6 (OMe), 20.6 (C-5″), 20.5* (C-5‴), 15.8 (C-4″), 15.7 (C-4‴), 15.1 (C-3).
β-sitosterol (6):1H NMR (600 MHz, CDCl3) of 6: δH 5.35 (1H, †, H-6), 3.51 (1H, m, H-3), 1.01 (3H, s, H-18), 0.92 (3H, d, J = 6.5 Hz, H-21), 0.84 (3H, t, J = 7.1 Hz, H-29), 0.83 (3H, d, J = 7 Hz, H-27), 0.81 (3H, d, J = 7 Hz, H-26), 0.68 (3H, s, H-19).
Stigmasterol (7)1H NMR (600 MHz, CDCl3) of 7: δH 5.35 (1H, †, H-6), 5.15 (2H, dd, J = 15.2/8.8 Hz, H-23), 5.02 (2H, dd (J = 15.3/8.8 Hz, H-22), 3.51 (1H, m, H-3).
Kaempferol 3-O-β-D-glucopyranoside (8):1H NMR (600 MHz, CD3OD) of 8: δH 8.03 (2H, dd, J = 6.9/1.9 Hz, H-2′, H-6′), 6.86 (2H, dd, J = 6.9/1.9 Hz, H-3′, H-5′), 6.24 (1H, d, J = 2 Hz, H-8), 6.08 (1H, d, J = 2 Hz, H-6), 5.12 (1H, d, J = 7.5 Hz, H-1″), 3.38–3.45 (2H, †, H-2″, H-3″), 3.30 (H, †, H-4″), 3.18 (H, m, H-5″), 3.67 (1H, dd, J = 11.9/2.4 Hz, H-6a″), 3.53 (1H, dd, J = 11.9/5.4 Hz, H-6b″); 13C NMR (150 MHz, CD3OD): δC 178.8 (C-4), 163 (C-7), 161.7 (C-5), 161.7 (C-4′), 158.9 (C-9), 158.3 (C-2), 135.3 (C-3), 132.2 (C-2′/C-6′), 122.9 (C-1′), 116.2 (C-3′/C-5′), 104.1 (C-10), 104.6 (C-1″), 101.2 (C-6), 96.2 (C-8), 78.4 (C-3″), 78.1 (C-5″), 75.8 (C-2″), 71.3 (C-4″), 61.6 (C-6″).
Kaempferol-3-O-α-rhamnopyranoside (9):1H NMR (600 MHz, CD3OD) of 9: δH 7.76 (2H, d, J = 8.8 Hz, H-2′, H-6′), 6.93 (2H, d, J = 8.8 Hz; H-3′, H-5′), 6.36 (1H, d, J = 1.7 Hz, H-8), 6.19 (1H, d, J = 1.8 Hz, H-6), 5.38 (1H, d, J = 1.5 Hz, H-1″), 4.22 (1H, m, H-2″), 3.32–3.34 (2H, †, H-4″, H-5″), 0.92 (3H, d, J = 5,9 Hz, H-6″); 13C (150 MHz, CD3OD): δC 178.2 (C-4), 164.7 (C-7), 161,7 (C-5), 160.1 (C-4′), 157.8 (C-9), 157.1 (C-2), 134.8 (C-3), 130.5 (C-2′, C-6′), 121.2 (C-1′), 115.2 (C-3′, C-5′), 104.4 (C-10), 102.1 (C-1″), 98.5 (C-6), 93.4 (C-8), 71.8(C-4″), 70.7 (C-3″), 70.5 (C-2″), 70.4 (C-5″), 16.2 (CH3).
Quercetin-3-O-β-glucopyranoside (10):1H NMR (600 MHz, CD3OD) of 10: δH 7.9 (1H, d, J = 2.1 Hz, H-2′), 7.45 (1H, dd, J = 8.5/2.1 Hz, H-6′), 6.82 (1H, d, J = 8.5 Hz, H-5′), 6.39 (1H, d, J = 2.1 Hz, H-8), 6.16 (1H, d, J = 2.0 Hz, H-6), 5.13 (1H, d, J = 7.3 Hz, H-1″), 3.63 (1H, dd, J = 11.8/2.5 Hz, H-6a″), 3.47 (1H, dd, J = 11.8/5.4 Hz, H-6b″), 3.32–3.38 (2H, †, H-2″, H-3″), 3.26 (1H, m, H-4″), 3.22 (1H, m, H-5″); 13C NMR (150 MHz, CD3OD) δC 179.2 (C-4), 165.4 (C-7), 162.9 (C-5), 158.9 (C-2), 158.0 (C-9), 149.3 (C-4′), 145.3 (C-3′), 135.7 (C-3), 124.1 (C-1′), 122.7 (C-6′), 118.1 (C-2′), 115.9 (C-5′), 105.4 (C-10), 105.1 (C-1″), 99.8 (C-6), 94.7 (C-8), 78.1 (C-3″), 77.8 (C-5″), 75.5 (C-2″), 71.0 (C-4″), 62.8 (C-6″).
2,4-dihydroxy benzoic acid (11): 1H NMR (600 MHz, CD3OD) of 11: δH 7.65 (1H, d, J = 8.5 Hz, H-6), 6.21 (1H, dd, J = 8.5/2.4 Hz, H-5), 6.18 (1H, d, J = 2.3 Hz, H-3); 13C NMR (150 MHz, CD3OD): δC 164.3 (C-2 or C-4), 162.9 (C-2 or C-4), 133.0 (C-6), 112.4 (C-1), 108.9 (C-5), 102.9 (C-3).
†Unclear due to overlapping.
*Signals of laserine and 2-epilaserine are interchangeable due to the similarity of the signals.
3.2. Antimicrobial activities and antioxidant capacities of the extracts
The chloroform extract of the aerial parts from F. caspica showed the highest antibacterial activity against gram-positive bacteria (Enterococcus faecalis and Staphylococcus aureus) with the MIC value of 32 and 64 μg/mL (Table 1), respectively.
Table 1.
Antimicrobial activities of F. caspica and F. halophila extracts (MIC, μg/mL).
| Bacteria |
Fungi |
||||||
|---|---|---|---|---|---|---|---|
|
S. aureus ATCC 29,213 |
E. faecalis ATCC29212 |
P. aeruginosa ATCC27853 |
E. coli ATCC 25,922 |
C. parapsilosis ATCC 90,018 |
C. krusei ATCC6258 |
C. albicans ATCC 90,028 |
|
| FC/AP/CHCl3 | 32 | 64 | 512 | 512 | 128 | 256 | 256 |
| FC/AP/MeOH | 1024 | 512 | 512 | 512 | 256 | 128 | 256 |
| FC/AP/EtOAc | 512 | 512 | 1024 | 1024 | 256 | 256 | 128 |
| Gentamicin | 0.12 | 8 | 1 | 0.5 | – | – | – |
| Fluconazole | – | – | – | – | 0.5 | 16 | 0.5 |
FC: Ferula caspica; AP: Aerial parts; CHCl3: Chloroform extract; MeOH: Methanolic extract; EtOAc: Ethyl acetate extract.
Bold value indicates FC/AP/CHCl₃ showed the highest antibacterial activity against S. aureus and E. faecalis
The ethyl acetate extract from the aerial parts of F. caspica exhibited the highest antioxidant activity in all test assays with the value of 177.23 ± 1.17 mg gallic acid equivalent/g extract in CUPRAC assay and 268.28 ± 1.84 mg Trolox equivalent/g extract in ABTS radical cation scavenging assay as well as 207.38 ± 7.42 mg quercetin equivalent/g extract in FRAP assay (Table 2). In addition, the same extract was found to have the highest total flavonoid and total phenolic content; 97.66 ± 1.89 mg quercetin equivalent/g extract and 214.04 ± 3.21 gallic acid equivalent/g extract (Table 2), respectively.
Table 2.
Antioxidant capacities of F. caspica and F. halophila extracts.
| Extracts | CUPRAC (mg GA/g extract) |
ABTS (mg Trolox/g extract) |
FRAP (mg Quercetin/g extract) |
Total phenol (mg GA/g extract) |
Total flavonoid (mg Quercetin/g extract) |
|---|---|---|---|---|---|
| FC/AP/CHCl3 | 42.64 ± 5.78 | 88.57 ± 1.24 | 12.94 ± 0.55 | 46.51 ± 2.03 | 8.17 ± 0.34 |
| FC/AP/MeOH | 53.75 ± 4.48 | 78.66 ± 3.07 | 58.65 ± 0.99 | 59.16 ± 3.42 | 12.14 ± 1.67 |
| FC/AP/EtOAc | 177.23 ± 1.17 | 268.28 ± 1.84 | 207.38 ± 7.42 | 214.04 ± 3.21 | 97.66 ± 1.89 |
FC: Ferula caspica; AP: Aerial parts; CHCl3: Chloroform extract; MeOH: Methanolic extract; EtOAc: Ethyl acetate extract; GA: Gallic acid; values statistically different at p < 0.05.
Bold value indicates FC/AP/EtOAc was the most active extract in all antioxidant capacity test assays
4. Discussion
In the literature, the antioxidant capacity of F. caspica has not been reported, previously, and there aren’t any recent studies on this species. To evaluate the antioxidant capacities of the extracts, ABTS radical cation scavenging activity, CUPRAC, and FRAP assays were tested. These methods are based on an electron transfer reaction resulted in a color change, the degree of which is related to the antioxidant concentration (Huang et al., 2005). ABTṠ+, Cu(II) and Fe(III) are reduced by acquiring an electron from antioxidant in corresponding assays (Re et al., 1999, Apak et al., 2004, Berker et al., 2007). According to the literature, phenolics/flavonoids are well known with their antioxidant activities varying by structural changes (Rice-Evans et al., 1997). Dehghan et al. (2007) determined that the total phenolic contents of hexane, diethyl ether, ethyl acetate, and methanol extracts of the aerial parts and roots of F. szovitsiana were correlated with DPPH radical scavenging activities and FRAPs. Thereby, the high antioxidant capacity of the ethyl acetate extract of F. caspica is accordant with the high phenolic/flavonoid content and the literature findings (Rice-Evans et al., 1997, Dehghan et al., 2007).
The antimicrobial activity of F. caspica has not been reported so far. Tamemoto et al. (2001) determined the significant antimicrobial activities of sesquiterpenes isolated from F. kuhistanica against methicillin-sensitive and methicillin-resistant S. aureus. Among the petroleum ether, hexane, hot/cold water and ethanol extracts of F. asafoetida, the hexane extract showed the highest antimicrobial activity and apolar extracts were found to be more active than the polar extracts (Bhatnager et al., 2015). The essential oil from the seeds of F. tunetana inhibited the Gram (+)/Gram (−) bacteria and phenylpropanoid-rich essential oil of the underground parts of the F. heuffelii exhibited antimicrobial activity against Gram (+) bacteria and fungi (Pavlović et al., 2012, Znati et al., 2017). The previous reports on F. ferulioides, Liu et al., 2013, Liu et al., 2015) tested the antimicrobial activities of isolated acetophenone and furocoumarin sesquiterpenes, including compounds 1–3. Compounds 1–3 showed antimicrobial activity against tetracycline-resistant S. aureus strain with the MIC values of 2 µg/mL, 2 µg/mL and 4 µg/mL, respectively. Therefore, the highest activity of the chloroform extract can be attributed to isolated compounds 1–5.
5. Conclusion
This is the first report of antioxidant capacity and antimicrobial activity of F. caspica M Bieb. In this research, eleven secondary metabolites were isolated from chloroform (1–7) and ethyl acetate extracts (8–11). The compounds 1, 2, 3, 4, 5, 6, 7 and 11 have been previously isolated from several Ferula species, while three flavonoid derivatives (8–10) were reported for the first time in Ferula species obtained from the ethyl acetate extract, which was the most active extract for antioxidant activity.
Acknowledgments
Acknowledgement
The authors thank Dr. Barış Özüdoğru, Hacettepe University, Faculty of Science, Department of Botany, Ankara, Turkey for supporting the collecting plant material and Prof. Dr. Hayri Duman, Gazi University, Faculty of Science, Department of Botany, Ankara, Turkey, for authentification of the plant specimen.
Funding
This study was supported by Hacettepe University Scientific Research Projects Coordination Unit. Project Number: 014 D03 301 002-552).
Footnotes
Peer review under responsibility of King Saud University.
References
- Abd E.-R.M.H., Wu Y.-C., Chang F.-R. Sesquiterpene coumarins from Ferula foetida. J. Chin. Chem. Soc. 2007;54(1):235–238. [Google Scholar]
- Ahmed A.A. Daucanes and other constituents from Ferula sinica. Phytochemistry. 1991;30(4):1207–1210. [Google Scholar]
- Alkhatib R., Hennebelle T., Joha S., Roumy V., Guezel Y., Biabiany M., Idziorek T., Preudhomme C., Quesnel B., Sahpaz S., Bailleul F. Humulane and germacrane sesquiterpenes from Ferula lycia. J. Nat. Prod. 2010;73(4):780–783. doi: 10.1021/np900827a. [DOI] [PubMed] [Google Scholar]
- Altundag E., Ozturk M. Ethnomedicinal studies on the plant resources of east Anatolia, Turkey. Proc. Soc. Behav. Sci. 2011;19:756–777. [Google Scholar]
- Apak R., Guclu K., Ozyurek M., Karademir S.E. Novel total antioxidant capacity index for dietary polyphenols and vitamins C and E, using their cupric ion reducing capability in the presence of neocuproine: CUPRAC method. J. Agric. Food Chem. 2004;52(26):7970–7981. doi: 10.1021/jf048741x. [DOI] [PubMed] [Google Scholar]
- Barrero A.F., Herrador M.M., Arteaga P. Sesquiterpenes and phenylpropanoids from Seseli vayredanum. Phytochemistry. 1992;31(1):203–207. [Google Scholar]
- Berker K.I., Güçlü K., Tor İ., Apak R. Comparative evaluation of Fe (III) reducing power-based antioxidant capacity assays in the presence of phenanthroline, batho-phenanthroline, tripyridyltriazine (FRAP), and ferricyanide reagents. Talanta. 2007;72(3):1157–1165. doi: 10.1016/j.talanta.2007.01.019. [DOI] [PubMed] [Google Scholar]
- Bhatnager R., Rani R., Dang A.S. Antibacterial activity of Ferula asafoetida: a comparison of red and white type. J. App. Biol. Biotech. 2015;3(2):18–21. [Google Scholar]
- Chang C.C., Yang M.H., Wen H.M., Chern J.C. Estimation of total flavonoid content in propolis by two complementary colorimetric methods. J. Food Drug Anal. 2002;10(3):178–182. [Google Scholar]
- Chaturvedula V.S.P., Prakash I. Isolation of stigmasterol and β-sitosterol from the dichloromethane extract of Rubus suavissimus. Int. Curr. Pharm. J. 2012;1(9):239–242. [Google Scholar]
- Chen B., Takaishi Y., Kawazoe K., Tamemoto K., Honda G., Ito M., Takeda Y., Kodzhimatov O.K., Ashurmetov O. Farnesyl hydroxybenzoic acid derivatives from Ferula kuhistanica. Chem. Pharm. Bull. 2001;49(6):707–710. doi: 10.1248/cpb.49.707. [DOI] [PubMed] [Google Scholar]
- Correia S.J., David J.M., Silva E.P., David J.P., Lopes L.M.X., Guedes M.L.S. Flavonóides, norisoprenóides e outros terpenos das folhas de Tapirira guianensis. Flavonoids, norisoprenoids and other terpenes from leaves of Tapirira guianensisQuím. Nova. 2008;31:2056–2059. (Portuguese) [Google Scholar]
- Dastan D., Salehi P., Gohari A.R., Ebrahimi S.N., Aliahmadi A., Hamburger M. Bioactive sesquiterpene coumarins from Ferula pseudalliacea. Planta Med. 2014;80(13):1118–1123. doi: 10.1055/s-0034-1382996. [DOI] [PubMed] [Google Scholar]
- Dehghan G., Shafiee A., Ghahremani M.H., Ardestani S.K., Abdollahi M. Antioxidant potential of various extracts from Ferula szovitsiana. in relation to their phenolic content. Pharm. Biol. 2007:691–699. [Google Scholar]
- Demirci S., Özhatay N. An ethnobotanical study in Kahramanmaraş (Turkey); wild plants used for medicinal purpose in Andırın, Kahramanmaraş. Turk. J. Pharm. Sci. 2012;9(1):75–92. [Google Scholar]
- Duman H., Sagiroglu M. A new species of Ferula (Apiaceae) from South Anatolia, Turkey. Bot. J. Linn. Soc. 2005;147(3):357–361. [Google Scholar]
- Galal A.M., Abourashed E.A., Ross S.A., ElSohly M.A., Al-Said M.S., El-Feraly F.S. Daucane sesquiterpenes from Ferula hermonis. J. Nat. Prod. 2001;64(3):399–400. doi: 10.1021/np000526x. [DOI] [PubMed] [Google Scholar]
- Güneş F., Özhatay N. An ethnobotanical study from Kars (Eastern) Turkey. Biol. Divers. Conserv. 2011;4(1):30–41. [Google Scholar]
- Güzel Y., Güzelşemme M., Miski M. Ethnobotany of medicinal plants used in Antakya: a multicultural district in Hatay Province of Turkey. J. Ethnopharmacol. 2015;174:118–152. doi: 10.1016/j.jep.2015.07.042. [DOI] [PubMed] [Google Scholar]
- Han J.-T., Bang M.-H., Chun O.-K., Kim D.-O., Lee C.-Y., Baek N.-I. Flavonol glycosides from the aerial parts of Aceriphyllum rossii and their antioxidant activities. Arch. Pharm. Res. 2004;27(4):390–395. doi: 10.1007/BF02980079. [DOI] [PubMed] [Google Scholar]
- Huang D., Ou B., Prior B. The chemistry behind antioxidant capacity assays. J. Agric. Food Chem. 2005;53(6):1841–1856. doi: 10.1021/jf030723c. [DOI] [PubMed] [Google Scholar]
- Iranshahi M., Hosseini S.T., Shahverdi A.R., Molazade K., Khan S.S., Ahmad V.U. Diversolides A-G, guaianolides from the roots of Ferula diversivittata. Phytochemistry. 2008;69(15):2753–2757. doi: 10.1016/j.phytochem.2008.08.009. [DOI] [PubMed] [Google Scholar]
- Iranshahi M., Kalategi F., Sahebkar A., Sardashti A., Schneider B. New sesquiterpene coumarins from the roots of Ferula flabelliloba. Pharm. Biol. 2010;48(2):217–220. doi: 10.3109/13880200903019226. [DOI] [PubMed] [Google Scholar]
- Iranshahi M., Rezaee R., Sahebkar A., Bassarello C., Piacente S., Pizza C. Sesquiterpene coumarins from the fruits of Ferula badrakema. Pharm. Biol. 2009;47(4):344–347. [Google Scholar]
- Isaka K., Nagatsu A., Ondognii P., Zevgeegiin O., Gombosurengyin P., Davgiin K., Kojima K., Ogihara Y. Sesquiterpenoid derivatives from Ferula ferulaeoides [correction of ferulioides]. V. Chem. Pharm. Bull. 2001;49(9):1072–1076. doi: 10.1248/cpb.49.1072. [DOI] [PubMed] [Google Scholar]
- Kojima K., Isaka K., Purev O., Jargalsaikhan G., Suran D., Mizukami H., Ogihara Y. Sesquiterpenoid derivatives from Ferula ferulioides. Chem. Pharm. Bull. 1998;46(11):1781–1784. [Google Scholar]
- Kojima K., Isaka K., Ondognii P., Zevgeegiin O., Gombosurengyin P., Davgiin K., Mizukami H., Ogihara Y. Sesquiterpenoid derivatives from Ferula ferulioides. IV. Chem. Pharm. Bull. 2000;48(3):353–356. doi: 10.1248/cpb.48.353. [DOI] [PubMed] [Google Scholar]
- Kurimoto S., Suzuki K., Okasaka M., Kashiwada Y., Kodzhimatov O.K., Takaishi Y. New sesquiterpene lactone glucosides from the roots of Ferula varia. Phytochem. Lett. 2012;5(4):729–733. doi: 10.1248/cpb.c12-00350. [DOI] [PubMed] [Google Scholar]
- Lhuillier A., Fabre N., Cheble E., Oueida F., Maurel S., Valentin A., Fouraste I., Moulis C. Daucane Sesquiterpenes from Ferula hermonis. J. Nat. Prod. 2005;68(3):468–471. doi: 10.1021/np049652h. [DOI] [PubMed] [Google Scholar]
- Li G., Li X., Cao L., Zhang L., Shen L., Zhu J., Wang J., Si J. Sesquiterpene coumarins from seeds of Ferula sinkiangensis. Fitoterapia. 2015;103:222–226. doi: 10.1016/j.fitote.2015.03.022. [DOI] [PubMed] [Google Scholar]
- Li G., Wang J., Li X., Cao L., Lv N., Chen G., Zhu J., Si J. Two new sesquiterpene coumarins from the seeds of Ferula sinkiangensis. Phytochem. Lett. 2015;13:123–126. doi: 10.1016/j.fitote.2015.03.022. [DOI] [PubMed] [Google Scholar]
- Liu T., Wang S., Xu L., Fu W., Gibbons S., Mu Q. Sesquiterpenoids with Anti-MDR Staphylococcus aureus activities from Ferula ferulioides. Chem. Biodivers. 2015;12(4):599–614. doi: 10.1002/cbdv.201400150. [DOI] [PubMed] [Google Scholar]
- Liu T., Osman K., Kaatz G.W., Gibbons S., Mu Q. Antibacterial sesquiterpenoid derivatives from Ferula ferulaeoides. Planta Med. 2013;79(8):701–706. doi: 10.1055/s-0032-1328461. [DOI] [PubMed] [Google Scholar]
- Meng H., Li G., Huang J., Zhang K., Wang H., Wang J. Sesquiterpene coumarin and sesquiterpene chromone derivatives from Ferula ferulaeoides (Steud.) Korov. Fitoterapia. 2013;86:70–77. doi: 10.1016/j.fitote.2013.02.002. [DOI] [PubMed] [Google Scholar]
- Meng H., Li G., Huang J., Zhang K., Wei X., Ma Y., Zhang C., Wang J. Sesquiterpenoid derivatives from Ferula ferulaeoides (Steud.) Korov. Phytochemistry. 2013;86:151–158. doi: 10.1016/j.phytochem.2012.10.013. [DOI] [PubMed] [Google Scholar]
- Miski M., Jakupovic J. Daucane esters from Ferula rigidula. Phytochemistry. 1990;29(1):173–178. [Google Scholar]
- Miski M., Mabry T.J. New daucane esters from Ferula tingitana. J. Nat. Prod. 1986;49(4):657–660. doi: 10.1021/np50046a016. [DOI] [PubMed] [Google Scholar]
- Miski M., Mabry T.J., Saya O. New daucane and germacrane esters from Ferula orientalis var. orientalis. J. Nat. Prod. 1987;50(5):829–934. doi: 10.1021/np50053a009. [DOI] [PubMed] [Google Scholar]
- Miski M., Ulubelen A., Lee E., Mabry T.J. Sesquiterpene-coumarin ethers of Ferula tingitana. J. Nat. Prod. 1985;48(2):326–327. doi: 10.1021/np50038a024. [DOI] [PubMed] [Google Scholar]
- Mükemre M., Behçet L., Çakilcioğlu U. Ethnobotanical study on medicinal plants in villages of Çatak (Van-Turkey) J. Ethnopharmacol. 2015;166:361–374. doi: 10.1016/j.jep.2015.03.040. [DOI] [PubMed] [Google Scholar]
- Oyaizu M. Studies on products of browning reactions-antioxidative activities of products of browning reaction prepared from glucosamine. Jpn. J. Nutr. 1986;44:307–315. [Google Scholar]
- Pavlović I., Petrović S., Radenković M., Milenković M., Couladis M., Branković S., Drobac M., Niketić M. Composition, antimicrobial, antiradical and spasmolytic activity of Ferula heuffelii Griseb. ex Heuffel (Apiaceae) essential oil. Food Chem. 2012;130(2):310–315. [Google Scholar]
- Peşmen H. Ferula L. In: Davis P.H., editor. Flora of Turkey and the East Aegean Islands. University Press; Edinburgh: 1972. pp. 440–453. [Google Scholar]
- Pimenov M.G., Kljuykov E.V. Ferula divaricata (Umbelliferae), a new species from Central Anatolia, Turkey. Phytotaxa. 2013;99(1):35–39. doi: 10.11646/phytotaxa.99.1.2. [DOI] [Google Scholar]
- Pimenov M.G., Leonev M.V. The Asian Umbelliferae biodiversity database (ASIUM) with particular reference to South-West Asian taxa. Turk. J. Botany. 2004;28:139–145. [Google Scholar]
- Re R., Pellegrini N., Proteggente A., Pannala A., Yang M., Rice-Evans C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999;26(9–10):1231–1237. doi: 10.1016/s0891-5849(98)00315-3. [DOI] [PubMed] [Google Scholar]
- Rice-Evans C.A., Miller N.J., Paganga G. Antioxidant properties of phenolic compounds. Trends Plant Sci. 1997;2(4):152–159. [Google Scholar]
- Sağıroğlu M. Gazi Üniversitesi; Ankara: 2005. Türkiye Ferula L. (Umbelliferae) cinsi'nin revizyonu [Revision of Turkish Ferula L. (Umbelliferae)] [dissertation] [Google Scholar]
- Sagiroglu M., Duman H. Ferula mervynii (Apiaceae), a distinct new species from North-East Anatolia, Turkey. Bot. J. Linn. Soc. 2007;153(3):357–362. [Google Scholar]
- Sagiroglu M., Duman H. Ferula brevipedicellata and F. duranii (Apiaceae), two new species from Anatolia, Turkey. Ann. Bot. Fenn. 2010;47(4):293–300. [Google Scholar]
- Sağıroğlu M., Duman H. Are Ferula tenuissima and F. amanicola distinct species or not? Biol. Divers. Conserv. 2014;7(3):74–77. [Google Scholar]
- Scott K.N. Carbon-13 nuclear magnetic resonance of biologically important aromatic acids. I. Chemical shifts of benzoic acid and derivatives. J. Am. Chem. Soc. 1972;94(24):8564–8568. doi: 10.1021/ja00779a045. [DOI] [PubMed] [Google Scholar]
- Slinkard K., Singleton V.L. Total phenol analysis: automation and comparison with manual methods. Am. J. Enol. Vitic. 1977;28(1):49–55. [Google Scholar]
- Tamemoto K., Takaishi Y., Chen B., Kawazoe K., Shibata H., Higuti T., Honda G., Ito M., Takeda Y., Kodzhimatov O.K., Ashurmetov O. Sesquiterpenoids from the fruits of Ferula kuhistanica and antibacterial activity of the constituents of F. kuhistanica. Phytochemistry. 2001;58(5):763–767. doi: 10.1016/s0031-9422(01)00307-7. [DOI] [PubMed] [Google Scholar]
- Yaqoob U., Nawchoo I.A. Distribution and taxonomy of Ferula L.: a review. Res. Rev. J. Bot. 2016;5(3):15–23. [Google Scholar]
- Wayne P. 8th ed., M 07-A8 ed. Clinical and Laboratory Standards Institute; 2008. Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria that Grow Aerobically: Approved Standard. [Google Scholar]
- Wayne P. 3rd ed., M 27-A3 ed. Clinical and Laboratory Standards Institute; 2008. Reference Method for Broth Dilution Antifungal Susceptibility Testing of Yeasts: Approved Standard. [Google Scholar]
- Yang J.-R., An Z., Li Z.-H., Jing S., Qin H.-L. Sesquiterpene coumarins from the roots of Ferula sinkiangensis and Ferula teterrima. Chem. Pharm. Bull. 2006;54(11):1595–1598. doi: 10.1248/cpb.54.1595. [DOI] [PubMed] [Google Scholar]
- Znati M., Ben Jannet H., Cazaux S., Souchard J.P., Skhiri F.H., Bouajila J. Antioxidant, 5-lipoxygenase inhibitory and cytotoxic activities of compounds isolated from the Ferula lutea flowers. Molecules. 2014;19(10):16959–16975. doi: 10.3390/molecules191016959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Znati, M., Filali, I., Jabrane, A., Casanova, J., Bouajila, J., Ben Jannet, H., 2017. Chemical composition and in vitro evaluation of antimicrobial, antioxidant and antigerminative properties of the seed oil from the Tunisian endemic Ferula tunetana Pomel ex Batt. 10.1002/cbdv.201600116. [DOI] [PubMed]