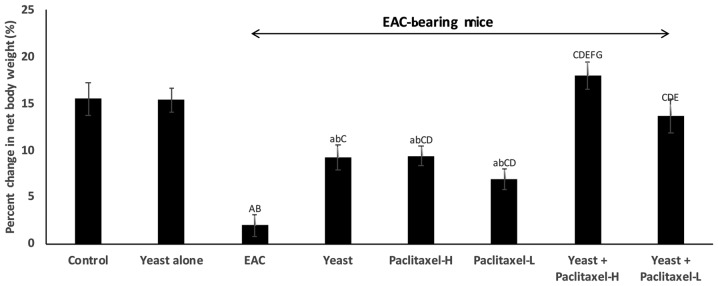
Figure 1.
Effect of yeast and/or paclitaxel on body weight change (g). Data are expressed as mean ± SE of 10 mice/group). aP<0.05 and AP<0.01, significantly different from the normal control untreated group. bP<0.05 and BP<0.01, significantly different from the control treated with yeast group. CP<0.01, significantly different from the EAC group. DP<0.01, significantly different from the yeast-treated EAC group. EP<0.01, significantly different from the paclitaxel-H-treated EAC group. FP<0.01, significantly different from the paclitaxel-L-treated EAC group. GP<0.01, significantly different from the yeast + paclitaxel-H-treated EAC group. Net final body weight = (final body weight - tumor weight). Body weight change = (net final body weight - initial body weight). EAC, Ehrlich ascites carcinoma. Groups: EAC, mice bearing tumors receiving intratumoral (i.t.) injections of PBS; Yeast, mice bearing tumors receiving i.t. injections of yeast (1×107 cells/ml); paclitaxel-H, mice bearing tumors receiving paclitaxel at a high dose (10 mg/kg); paclitaxel-L, mice bearing tumors receiving paclitaxel at a low dose (2 mg/kg); Yeast + Paclitaxel-H, mice bearing tumors receiving yeast plus paclitaxel-H; Yeast + Paclitaxel-L, mice bearing tumors receiving yeast plus paclitaxel-L.