Figure 1.
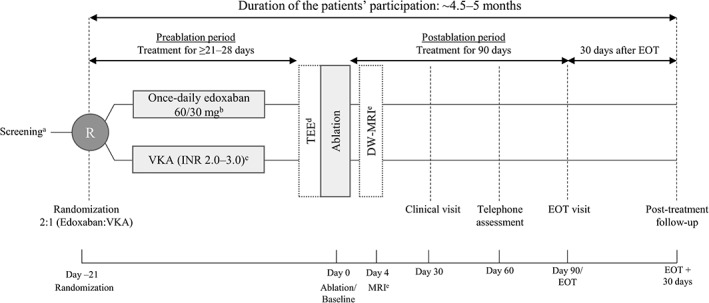
Schematic diagram of study design, including 21 to 28 days of anticoagulation with edoxaban or VKA during the pre‐ablation period, 90 days of post‐ablation treatment, and a 30‐day follow‐up. Abbreviations: CrCl, creatinine clearance; DW‐MRI, diffusion‐weighted magnetic resonance imaging; EOT, end of treatment; INR, international normalized ratio; MRI, magnetic resonance imaging; P‐gp, P‐glycoprotein; TEE, transesophageal echocardiography; VKA, vitamin K antagonist. aScreening and randomization visits may be combined if the inclusion/exclusion criteria can be checked based on health records and actual laboratory results with sufficient accuracy and the patient received informed consent ≥1 day prior to randomization. bPatients will be dose‐reduced to 30 mg edoxaban once daily if they meet any of the following: CrCl 15–50 mL/min, body weight ≤ 60 kg, or being treated with select P‐gp inhibitors. cAll patients randomized to VKA will be required to be in the INR range of 2.0 to 3.0 for the last 10 days prior to the ablation procedure. dTEE in all patients 1 day prior to or on the morning of the ablation procedure. If intracardiac clots are identified, then the ablation procedure will not be performed and the patients will be switched from study medication to standard of care and will enter the 30‐day follow‐up period. eDW‐MRI, diffusion‐weighted magnetic resonance imaging
