Abstract
Background
Advanced practice providers (APPs) can fill care gaps created by physician shortages and improve adherence/compliance with preventive ASCVD interventions.
Hypothesis
APPs utilizing guideline‐based algorithms will more frequently escalate ASCVD risk factor therapies.
Methods
We retrospectively reviewed data on 595 patients enrolled in a preventive cardiology clinic (PCC) utilizing APPs compared with a propensity‐matched cohort (PMC) of 595 patients enrolled in primary‐care clinics alone. PCC patients were risk‐stratified using Framingham Risk Score (FRS) and coronary artery calcium scoring (CACS).
Results
Baseline demographics were balanced between the groups. CACS was more commonly obtained in PCC patients (P < 0.001), resulting in reclassification of 30.6% patients to a higher risk category, including statin therapy in 26.6% of low‐FRS PCC patients with CACS ≥75th MESA percentile. Aspirin initiation was higher for high and intermediate FRS patients in the PCC (P < 0.001). Post‐intervention mean LDL‐C, non–HDL‐C, and triglycerides (all P < 0.05) were lower in the PCC group. Compliance with appropriate lipid treatment was higher in intermediate to high FRS patients (P = 0.004) in the PCC group. Aggressive LDL‐C and non–HDL‐C treatment goals (<70 mg/dL, P = 0.005 and < 130 mg/dL, P < 0.001, respectively), were more commonly achieved in high‐FRS PCC patients. Median post‐intervention SBP was lower among intermediate and low FRS patients (P = 0.001 and P < 0.001, respectively). Cumulatively, this resulted in a reduction in median post‐intervention PCC FRS across all initial FRS risk categories (P < 0.001 for all).
Conclusions
APPs within a PCC effectively risk‐stratify and aggressively manage ASCVD risk factors, resulting in a reduction in post‐intervention FRS.
Keywords: Atherosclerosis, Blood Pressure Control and Regulation, Computed Tomography, General Clinical Cardiology/Adult, Imaging, Preventive Cardiology
1. INTRODUCTION
Atherosclerotic cardiovascular disease (ASCVD) remains the leading cause of mortality in the United States.1, 2 This high prevalence of disease, morbidity, and mortality continues to be observed despite significant advancements in ASCVD risk assessment and management. In the face of this high disease prevalence, data from the National Cardiovascular Data Registry Proactive Innovation and Clinical Excellence (NCDR PINNACLE) observed that 43.9% of cholesterol treatment–eligible primary‐prevention patients were receiving a statin medication and up to 35.9% were not receiving any lipid‐lowering therapy.3
Downstream costs associated with the care of patients presenting with ASCVD events are tremendous. However, more robust implementation of primary‐prevention therapies is complicated by the fact that the United States is in the midst of a shortage of primary‐care physicians and cardiologists.4 Advanced practice providers (APPs) may provide an opportunity to fill this vital gap in the healthcare delivery team to both expand access and relieve some burden from primary‐care managers.5, 6, 7 The appropriate utilization of APPs in a primary‐prevention, subspecialty clinic population has the possibility to positively impact adherence to guideline‐directed therapy, as it has been shown to do in secondary‐prevention, diabetes mellitus (DM), and heart failure populations previously.4, 8, 9
We sought to analyze the effectiveness of risk stratification, initiation of recommended medical therapies, and resultant changes in global ASCVD risk by APPs with indirect oversight by a cardiologist utilizing locally developed treatment algorithms based on published guidelines.
2. METHODS
2.1. Study population
A population of 595 patients without known ASCVD referred to a preventive cardiology clinic (PCC) at a single‐center military treatment facility from January 1, 2009, to December 31, 2013, was included in the study population. Baseline demographic data, initial and follow‐up laboratory and imaging data, and cardiovascular risk factors were abstracted. An age and risk‐factor propensity‐matched cohort (PMC) was derived in a 1:1 fashion from an initial population of 20 604 patients enrolled in internal medicine and family medicine clinics in the same healthcare system over the same time period.
2.2. The PCC
The PCC is embedded within the cardiology division and utilizes a clinical pharmacist, physician assistants, and a nurse practitioner supervised by a board‐certified cardiologist. The PCC accepts primary‐prevention adult patients from primary‐care clinics and other specialty‐care clinics within the local healthcare system. The APPs manage primary‐prevention medications and pursue smoking cessation working with a guideline‐based, locally developed algorithm utilizing Framingham Risk Score (FRS) and coronary artery calcium scoring (CACS). Treatment and follow‐up testing decisions are made by APPs independently.
2.3. Initial evaluation
Baseline evaluation obtained at the initial visit included a fasting lipid profile, fasting serum glucose, blood pressure (BP) measurements, and height/weight measurements. Cardiovascular risk factors as defined in PCC algorithms were as follows: smoking (active or prior >10 pack‐years), hypertension (HTN; previous diagnosis, active treatment with an antihypertensive medication, or a systolic blood pressure [SBP] ≥140 mm Hg), DM (previous diagnosis, active treatment with an oral antihyperglycemic or insulin, glycated hemoglobin [HbA1c] level ≥ 6.5%, or fasting serum glucose ≥126 mg/dL), and hyperlipidemia (previous diagnosis or active treatment with lipid‐lowering medication). Based on these risk‐factor definitions and initial laboratory testing, APPs perform risk‐factor counseling utilizing the National Cholesterol Education Program (NCEP) Adult Treatment Panel (ATP) III panel recommendations10, 11, 12.
2.4. Lipid‐lowering therapy
Initiation, escalation, or discontinuation of lipid‐lowering medication(s) was based on low‐density lipoprotein cholesterol (LDL‐C) targets as defined by the ATP III recommendations. All patients referred to the PCC were counseled on heart‐health dietary interventions. Lipid therapy escalation was defined as an increase in statin therapy intensity, initiation of statin therapy in untreated patients, or addition of a secondary lipid‐lowering medication. Lipid therapy de‐escalation was defined as a decrease in statin intensity or statin dose, or discontinuation of statin therapy.
2.5. CACS
The CACS studies were obtained using an electrocardiogram gated 128‐slice dual‐source computed tomography (CT) scanner (SOMATOM Definition Flash CT; Siemens, Erlangen, Germany). Foci of CAC were identified using semiautomatic commercial software (Vitrea 6.3 software; Vital Images, Minnetonka, MN). A total calcium score was derived using the Agatston scoring method, as was the Multi‐Ethnic Study of Atherosclerosis (MESA) percentile.13
2.6. Management of HTN
Patients were screened and treated for HTN in accordance with Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure (JNC 7) recommendations. Lifestyle‐modification counseling was performed on every patient. Antihypertensive therapy was initiated at the discretion of the treating APP per a locally developed protocol based on JNC 7 treatment recommendations; the goal BP was <140/90 mm Hg in all patients, except for patients with DM or chronic kidney disease (goal BP <130/80 mm Hg in these patients).
2.7. Management of DM
Patients with initial HbA1c levels >7.0% were considered for initiation of antihyperglycemic therapies. Recommended annual screening for microalbuminuria, peripheral neuropathy, and diabetic retinopathy was also performed. Patients achieving target HbA1c levels were monitored in 3‐ to 6‐month intervals, whereas patients with persistently elevated HbA1c levels were referred to a specialized DM care clinic within the endocrinology division for evaluation of insulin or other more advanced therapies.
2.8. Tobacco‐cessation counseling
All patients referred to the PCC were screened for tobacco use. Patients willing to quit were referred to a weekly tobacco‐cessation information class, as well as assessed for individualized intervention intensity level. Interventions ranged from a single counseling session without pharmacotherapy intervention to ≥6 counseling sessions, pharmacotherapeutic initiation, short‐interval clinic follow‐up, and referral to a weekly support group.
2.9. Follow‐up
Follow‐up FRS in all patients was performed based on data obtained 12 months (±6 months) from the initial clinic encounter. Changes in BP and lipids were calculated as percent changes from baseline laboratory and BP data, with a positive percentage representing a favorable change and a negative percentage indicating a negative change. Initial and follow‐up laboratory data within 6 months prior to the initial primary‐care visit were labeled as baseline data, and follow‐up laboratory data ≥3 months after the initial visit was abstracted and used to assess follow‐up therapy and laboratory changes.
2.10. Statistical analysis
Discrete variables were reported as proportions. Normally distributed continuous variables were reported as a mean ±SD, and non‐normal continuous data were reported as median (interquartile range). Statistical significance was defined at the <0.05 level for all analyses (2‐tailed). The PMC was derived utilizing Mahalanobis metrics matching. Between‐group comparisons of continuous variables was obtained using 1‐ and 2‐way ANOVA testing or Wilcoxon rank‐sum tests, as appropriate. Categorical variables were compared using the Pearson χ2 test. All data variables were analyzed using SPSS version 22 (IBM Corp., Armonk, NY).
3. RESULTS
3.1. Clinical characteristics
The baseline clinical characteristics for the PCC and PMC patient groups are shown in Table 1. The PCC group was more likely to be treated initially for HTN (P < 0.001). Otherwise, baseline demographics and cardiac risk factors were well balanced between the groups. The median FRS at initial evaluation was higher in the PCC cohort (15.9%) compared with the PMC patients (11.5%; P < 0.001). This was driven by more high‐FRS patients (P < 0.001) and fewer low‐FRS patients (P < 0.001) in the PCC cohort (Table 1).
Table 1.
Baseline characteristics
| PCC, N = 595 | PMC, N = 595 | P Value | |
|---|---|---|---|
| Age, y | 58.3 ± 10.0 | 57.9 ±10.9 | 0.480 |
| Male sex | 430 (72.3) | 430 (72.3) | 0.526 |
| HTN | 419 (70.4) | 428 (71.9) | 0.304 |
| Hyperlipidemia | 595 (100) | 595 (100) | 1.00 |
| DM | 152 (25.5) | 151 (25.4) | 0.500 |
| Smoker | 94 (15.8) | 96 (16.1) | 0.468 |
| AF | 6 (1.0) | 4 (<1.0) | 0.376 |
| Treatment for HTN | 392 (65.9) | 302 (50.8) | <0.001 |
| BP controlled | 458 (77) | 459 (77) | 0.500 |
| Initial SBP, mm Hg | 128 (120–140) | 128 (119–138) | 0.772 |
| BMI, kg/m2 | 29 (26–33) | 29.4 (26.3–32.8) | 0.653 |
| CV risk estimates | |||
| Initial FRS | 15.86 (9.11–26.58) | 11.53 (6.73–18.89) | <0.001 |
| High | 238 (40) | 135 (22.7) | <0.001 |
| Intermediate | 184 (31) | 208 (35) | 0.078 |
| Low | 173 (29.1) | 252 (42.4) | <0.001 |
| CACS obtained | 493 (82.9) | 65 (10.9) | <0.001 |
| CACS, AU | 131.56 ± 305.16 | 147.631 ± 420.35 | 0.704 |
| Initial lipid values, mg/dL | |||
| TC | 195 (164–221) | 194 (166–223) | 0.833 |
| LDL‐C | 109 (83–137) | 115 (91–143) | 0.036 |
| TG | 124 (81–191) | 117 (85–169) | 0.211 |
| HDL‐C | 48 (39–63) | 47 (40–57) | 0.575 |
| Non–HDL‐C | 140 (113–169) | 144 (117–170) | 0.309 |
| ASA prescription following initial evaluation | |||
| High FRS | 169 | 51 | <0.001 |
| Intermediate FRS | 106 | 64 | <0.001 |
| Low FRS | 62 | 32 | <0.001 |
| Lipid medications | |||
| Low‐intensity statin therapy | 51 (8.6) | 40 (6.7) | 0.275 |
| Moderate‐intensity statin therapy | 176 (29.6) | 169 (28.4) | 0.708 |
| High‐intensity statin therapy | 85 (14.3) | 59 (9.9) | 0.013 |
| Nonstatin lipid therapy only | 85 (14.3) | 33 (5.5) | <0.001 |
| No lipid therapy | 198 (33.2) | 294 (49.4) | <0.001 |
| Combination lipid therapya | 94 (15.8) | 24 (4.0) | <0.001 |
| Initial BP values/treatment | |||
| Treatment for HTN | 392 (65.9) | 302 (50.8) | <0.001 |
| SBP, mm Hg | 130 ± 16 | 129 ± 14 | 0.060 |
| Initial SBP well controlled | 458 (77.0) | 459 (77.1) | 1.000 |
Abbreviations: AF, atrial fibrillation; ASA, acetylsalicylic acid (aspirin); AU, Agatston units; BMI, body mass index; BP, blood pressure; CACS, coronary artery calcium score; CV, cardiovascular; DM, diabetes mellitus; FRS, Framingham Risk Score; HDL‐C, high‐density lipoprotein cholesterol; HTN, hypertension; IQR, interquartile range; LDL‐C, low‐density lipoprotein cholesterol; PCC, preventive cardiology clinic; PMC, propensity‐matched cohort; SBP, systolic blood pressure; SD, standard deviation; TC, total cholesterol; TG, triglycerides.
Data are presented as n (%), mean ± SD, or median (IQR).
Statin and nonstatin medication use.
3.2. CACS
Testing for CACS was obtained in 82.9% of PCC patients, compared with only 10.9% of patients in the PMC cohort (P < 0.001). PCC patients had a mean CACS of 131.56 ±305.16 AU, with a CACS of 0 AU seen among 39.0%, and 12.4% having a CACS >300 AU. Among high‐FRS patients, 38 (20.8%) had a CACS of 0 AU; among low‐FRS patients, 62 (41.3%) had a detectable CACS, of which 20 (13.3%) had a CACS >100 AU. In the low‐ and intermediate‐FRS groups, a total of 96 (30.6%) patients had a CACS that placed them into the 75th percentile for their age and sex, thus reclassifying them to a higher risk category (see Supporting Information, Figure 1, in the online version of this article).
Figure 1.
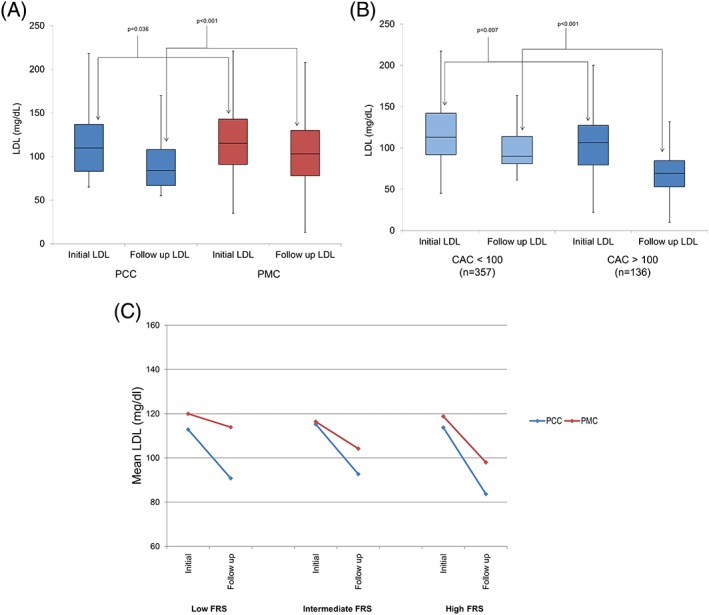
Observed LDL changes. (A) Median (IQR) initial and post‐intervention LDL‐C values in the PCC and PMC cohorts; (B) median (IQR) initial and post‐intervention LDL‐C values PCC patients who underwent CACS stratified by CACS < or > 100 arbitrary units; (C) mean LDL‐C changes post‐intervention in the PCC and PMC groups (P < 0.05 for follow‐up LDL‐C between all FRS groups). Abbreviations: CACS, coronary artery calcium scoring; FRS, Framingham Risk Score; IQR, interquartile range; LDL‐C, low‐density lipoprotein cholesterol; PCC, preventive cardiology clinic; PMC, propensity‐matched cohort
3.3. Utilization of aspirin
Overall, aspirin utilization among intermediate‐ and high‐FRS patients was higher than national trends in both cohorts (65.2% in PCC vs 33.5% in PMC). Aspirin prescription post‐intervention was higher for high‐ and intermediate‐FRS patients in the PCC (P < 0.001; Table 1).
3.4. Lipid management
Patients in the PCC had lower baseline LDL‐C (P = 0.036) and were more commonly on a high‐intensity statin (P = 0.013) and nonstatin lipid therapy (P < 0.001) when compared with the PMC (see Supporting Information, Figure 2, in the online version of this article). The remaining baseline lipid‐panel values were not different between the groups (Table 1). Initiation of lipid‐lowering therapy in treatment‐naïve patients was pursued in 64.6% of PCC patients, compared with 49.3% of PMC patients (P = 0.001; see Supporting Information, Figure 2, in the online version of this article). This difference was driven both by higher rates of appropriate treatment among intermediate‐ to high‐FRS patients (77.3% vs 60.6%; P = 0.004) and treatment of 26.6% of low‐FRS PCC patients with CACS >75th percentile per MESA database (see Supporting Information, Figure 2, in the online version of this article).
Figure 2.
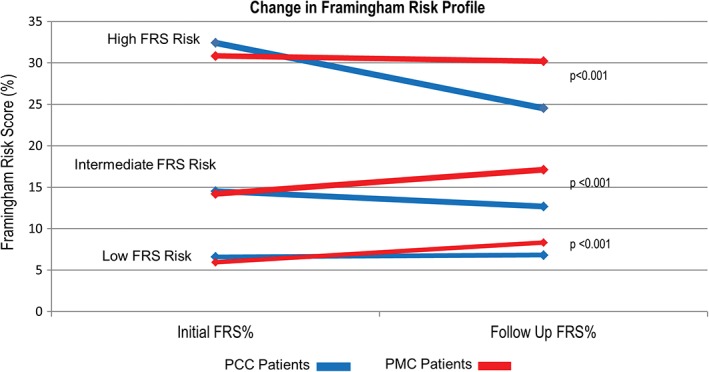
Changes in median FRS predicted 10‐year ASCVD risk at initial and post‐intervention in PCC and PMC groups. Abbreviations: ASCVD, atherosclerotic cardiovascular disease; FRS, Framingham Risk Score; PCC, preventive cardiology clinic; PMC, propensity‐matched cohort
Post‐intervention, median LDL‐C values were reduced in the PCC cohort compared with PMC patients (P < 0.001; Figure 1A). More high‐FRS PCC patients (Table 2) achieved an LDL‐C < 70 mg/dL (P = 0.005) and a non–high‐density lipoprotein cholesterol (non–HDL‐C) of <130 mg/dL (P < 0.001). Additionally, reduction in median LDL‐C (P = 0.030), non–HDL‐C (P = 0.001), and triglycerides (P = 0.009) was observed in the PCC cohort (Table 2).
Table 2.
Changes in clinical risk factors, medical treatment, and laboratory values
| High FRS | Intermediate FRS | Low FRS | |||||||
|---|---|---|---|---|---|---|---|---|---|
| PCC, n = 238 | PMC, n = 135 | P Value | PCC, n = 184 | PMC, n = 208 | P Value | PCC, n = 173 | PMC, n = 252 | P Value | |
| Changes in lipid profile | |||||||||
| LDL <70 mg/dL | 88 (36.9) | 32 (23.7) | 0.005 | 34 (18.5) | 52 (25) | 0.053 | 31 (17.9) | 71 (28.2) | 0.013 |
| TG <150 mg/dL | 161 (67.6) | 82 (60.7) | 0.112 | 48 (26.1) | 51 (24.5) | 0.480 | 120 (69.4) | 155 (61.5) | 0.024 |
| Non–HDL‐C <130 mg/dL | 181 (76.1) | 73 (54.0) | <0.001 | 50 (27.2) | 39 (18.8) | 0.055 | 162 (93.6) | 220 (87.3) | <0.001 |
| TC reduction, % | 15 (3.03, 31.63) | 9.94 (−8.1, 22.8) | 0.030 | 130 (70.7) | 121 (58.2) | 0.019 | 8.7 (−3.6, 21.0) | 1.0 (−13.5, 14.3) | <0.001 |
| LDL‐C reduction, % | 21 (1.25, 45.12) | 17 (−7.87, 35.63) | 0.030 | 159 (86.4) | 152 (73.1) | 0.004 | 15.5 (−4.0, 35.8) | 1.2 (−15.9, 22.6) | <0.001 |
| HDL‐C increase, % | 0.04 (−0.08, 0.16) | 0.04 (−0.06, 0.19) | 0.403 | 8.8 (−3.6, 26.3) | 2.0 (−9.9, 16.1) | 0.001 | 0.0 (−0.1, 0.1) | 0.0 (−0.1, 0.1) | 0.593 |
| Non–HDL‐C reduction, % | 21.5 (3.77, 42.5) | 14.7 (−7.3, 30.1) | 0.001 | 13.2 (−4.2, 35.2) | 9.1 (−11.8, 27.7) | 0.033 | 11.9 (−4.8, 32.7) | 1.2 (−19.2, 18.3) | <0.001 |
| TG reduction, % | 17.1 (−9.76, 38.4) | 5.98 (−24.0, 26.0) | 0.009 | 0.0 (−0.1, 0.1) | 0.0 (−0.1, 0.1) | 0.101 | 2.1 (−32.3, 27.4) | −15.3 (−57.5, 13.1) | <0.001 |
| Changes in BP | |||||||||
| SBP at follow‐up, mm Hg | 128 (122, 138) | 132 (123, 141) | 0.102 | 124 (116, 132) | 129 (119, 138.2) | 0.001 | 121 (111, 131) | 127 (118, 135) | <0.001 |
| SBP reduction, % | 5.5 (−1.47, 12.0) | 4.1 (−4.4, 11.95) | 0.237 | 0.8 (−2.2, 7.1) | 0.0 (−9.9, 7.8) | 0.035 | 0 (−6.2, 5.8) | −2.3 (−11, 4.8) | 0.022 |
| Changes in lipid medications | |||||||||
| No therapy | 20 (8.4) | 30 (22.2) | <0.001 | 21 (11.4) | 46 (22.1) | 0.005 | 43 (24.9) | 109 (43.3) | <0.001 |
| Combination therapya at follow‐up | 81 (34) | 5 (3.70) | <0.001 | 38 (31.1) | 9 (5.3) | <0.001 | 40 (23.1) | 10 (3.96) | <0.001 |
Abbreviations: BP, blood pressure; FRS, Framingham Risk Score; HDL‐C, high‐density lipoprotein cholesterol; IQR, interquartile range; LDL‐C, low‐density lipoprotein cholesterol; PCC, preventive cardiology clinic; PMC, propensity‐matched cohort; SBP, systolic blood pressure; TC, total cholesterol; TG, triglycerides.
Data are presented as n (%) or median (IQR); IQR presented as “n, n” to avoid confusion between dashes and minus signs in values <0.
Statin therapy with nonstatin therapy.
CACS‐stratified changes in median LDL‐C (Figure 2B) were also significant in that patients with a CACS >100 AU had a more dramatic reduction in LDL‐C post‐intervention than did those with CACS of <100 AU (P < 0.001). The algorithm‐driven risk‐factor management among PCC patients resulted in significant reductions in median FRS across all risk categories when compared with PMC patients (P < 0.001 for all groups; Figure 2C).
3.5. Management of BP
Baseline BP readings (Table 1) were well controlled (defined as SBP ≤120 mm Hg) in more than three‐quarters of the patients in both groups (P = 1.000). There was no difference in mean SBP between the 2 cohorts post‐intervention (126 ±13 mm Hg vs 129 ±14 mm Hg; P = 0.189) or the rate of well‐controlled SBP (P = 0.134). There was a substantial 4% to 5% reduction in SBP in the high‐FRS patients (Table 2) in both groups (P = 0.237 for between‐group difference). However, among the intermediate‐ and low‐FRS patients post‐intervention, significantly lower mean SBP was observed in the PCC cohort when compared with the PMC patients (P = 0.001 and P < 0.001, respectively), driven primarily by maintenance of stable BP readings from baseline compared with a higher proportion of patients with worsening in SBP readings post‐intervention in the PMC group (Table 2).
4. DISCUSSION
The use of APPs in a PCC utilizing guideline‐based local risk factor–modification algorithms, combined with routine utilization of CACS, resulted in higher rates of lipid‐lowering therapy initiation in treatment‐naïve patients, more frequent appropriate escalation of lipid‐lowering therapy, and more frequent use of combination lipid‐lowering therapy when compared with age‐ and risk factor–matched patients treated by primary‐care managers. Additionally, intermediate‐and high‐risk PCC patients were more commonly prescribed aspirin therapy, and mean SBP was lower following PCC intervention in intermediate‐ and low‐FRS PCC patients. This resulted in a significant global risk reduction, regardless of initial FRS category, in the PCC cohort compared with PMC patients. Additionally, frequent use of CACS as an individualized risk‐stratification approach identified a significant cohort of low‐FRS patients with CACS exceeding the 75th MESA percentile.
In the United States, team‐based care models comprising various combinations of cardiologists and APPs have been pioneered for the management of chronic cardiovascular conditions, ranging from chronic heart failure management to coronary artery disease and lipid‐management clinics.6, 9, 14, 15, 16, 17 Additionally, data from a primary‐care outreach network in Oregon demonstrated improved lipid monitoring and higher rates of lipid‐lowering prescriptions in patients with DM managed remotely by a clinical pharmacist‐physician team.18 Clinical pharmacist‐led care teams effectively manage HTN across multiple health systems within a primary‐care setting.19 The PCC model presented in this analysis differs from published data in several ways. Through the creation of algorithms of care, there was less ambiguity for APPs regarding escalation of medical care. Additionally, the breadth of ASCVD risk factors addressed within a single clinic is novel. Finally, the frequent, up‐front utilization of CACS to individualize ASCVD risk stratification allowed for a more patient‐centered approach to primary prevention and may have improved compliance with recommended therapies.
ASCVD events continue to be the primary cause of morbidity and mortality in the first world. Data suggest that both physicians and APPs practicing within cardiology clinics do not routinely prescribe recommended medical therapies for various ASCVD events in patients who meet guideline criteria to be offered therapy. Physicians and APPs were compliant with ASCVD medication interventions in approximately 12% of patients in a large PINNACLE NCDR registry.4 In our analysis, treatment in a PCC cohort resulted in 85.8% of patients eligible for lipid‐lowering therapy actually being on lipid‐lowering therapy, which is tremendously higher than reported compliance rates. More striking is the fact that compliance with lipid therapies was high at baseline in both cohorts (approximately 50%), thus demonstrating that this model is effective even in high‐compliance healthcare systems.
Costs associated with care in our 2 cohorts could not be calculated due to a lack of patient‐level billing data; however, the potential cost implications of improved ASCVD event prevention, in addition to direct patient care administered by APPs, has been demonstrated.20 Medicare reimburses for care administered by APPs at up to 85% of a physician's rate.21 Costs associated with long‐term management of patients following a ASCVD event are higher, as shown in DM populations, among others.22 Thus, higher rates of medication compliance observed in our PCC cohort would be assumed to lead to lower downstream event rates and reduction in direct costs due to APPs delivering the care and fewer hospitalizations/revascularization procedures. Additionally, indirect costs may decrease as a result of increased patient productivity, decreased days off work, and increased quality‐adjusted life‐years.
Utilization of CACS as part of the initial risk‐stratification strategy was very high within the PCC cohort, at nearly 83%. Although this degree of CACS exceeds the volume utilized in most clinical practices nationwide, there is abundant data that CACS incrementally improves risk assessment alone and as an adjunctive test to global risk scores.23, 24, 25 Among a cohort of the MESA population deemed statin ineligible, CACS reclassified 6.8% of patients upward, with a calculated number needed to screen of 14.7 to prevent a ASCVD event.26 Conversely, among statin candidates, CACS identified 44% of individuals with a CACS of 0 AU in whom statins were recommended but had an observed event rate of <0.5% per year.27 There are robust data supporting treatment based on CACS compared with a risk‐assessment strategy involving no imaging. The Early Identification of Subclinical Atherosclerosis by Noninvasive Imaging Research (EISNER) trial showed that use of CACS resulted in a lower FRS at 4 years of follow‐up when compared with no scanning.28 Additionally, a CACS‐based strategy was also associated with favorable changes in SBP (P = 0.02), LDL‐C (P = 0.04), and waist circumference (P = 0.01), without increased downstream medical testing.28 A recently published meta‐analysis found that, compared with risk‐assessment strategies not involving CACS, individuals found to have CAC were more likely to be started on aspirin, statin therapy, and antihypertensive medications or to have intensification of baseline medical therapy.29
4.1. Study limitations
Lipid‐lowering treatment algorithms reported on in this analysis are based on ATP III treatment guidelines utilizing FRS for global risk estimation; thus, applicability to current clinical practice may be lessened.10, 30, 31 Despite acceptable propensity matching for individual ASCVD risk factors between the groups, there was an observed baseline difference between median FRS between the groups. PMC patients were evaluated and treated by primary‐care managers with numerous other clinical metrics to address, in addition to ASCVD prevention. Thus, a singularly focused PCC model would be expected to perform well in comparison. Finally, complications resulting from more aggressive risk‐factor treatment, such as statin‐induced myalgias or bleeding relating to aspirin, were not tracked in this population. Therefore, no comment or conclusions can be made about the potential negative ramifications of more aggressive treatment in the PCC population.
5. CONCLUSION
A PCC staffed with APPs practicing under guideline‐based treatment algorithms can effectively risk‐stratify and aggressively treat patients with ASCVD risk with observed improvement in serum lipid panels and estimated global cardiovascular risk over an intermediate follow‐up period.
Conflicts of interest
The views expressed herein are those of the authors and do not reflect the official policy or position of Brooke Army Medical Center, the US Army Medical Department, the US Army Office of the Surgeon General, the Department of the Army, the Department of the Air Force, the Department of Defense or the U.S. government. The authors declare no potential conflicts of interest.
Supporting information
Figure S1. Distribution of Coronary Artery Calcium Scores (CACS) Among PCC patients (A) 10‐ year FRS predicted ASCVD risk among different CAC score groups (B) CACS distribution amongst risk groups. (C) MESA percentile distribution amongst patients with and without CACS >100 arbitrary units.
Figure S2. Lipid Lowering Therapies and Statin Intensity per FRS category Initial and at follow‐up post‐intervention amongst (A) PMC patients and (B) PCC patients. Panel C shows post‐intervention lipid therapy treatment data in treatment naïve patients in the PMC and PCC cohorts. Panel D depicts overall lipid therapy changes amongst PCC and
Fentanes E, Vande Hei AG, Holuby RS, et al. Treatment in a preventive cardiology clinic utilizing advanced practice providers effectively closes atherosclerotic cardiovascular disease risk‐management gaps among a primary‐prevention population compared with a propensity‐matched primary‐care cohort: A team‐based care model and its impact on lipid and blood pressure management. Clin Cardiol. 2018;41:817–824. 10.1002/clc.22963
[The copyright line for this article was changed on 30‐July 2019 after original online publication]
REFERENCES
- 1. Mozaffarian D, Benjamin EJ, Go AS, et al; American Heart Association Statistics Committee; Stroke Statistics Subcommittee . Executive summary: Heart Disease and Stroke Statistics—2016 update: a report from the American Heart Association. Circulation. 2016;133:447–454. [DOI] [PubMed] [Google Scholar]
- 2. Domanski M, Lloyd‐Jones D, Fuster V, et al. Can we dramatically reduce the incidence of coronary heart disease? Nat Rev Cardiol. 2011;8:721–725. [DOI] [PubMed] [Google Scholar]
- 3. Maddox TM, Borden WB, Tang F, et al. Implications of the 2013 ACC/AHA cholesterol guidelines for adults in contemporary cardiovascular practice: insights from the NCDR PINNACLE registry. J Am Coll Cardiol. 2014;64:2183–2192. [DOI] [PubMed] [Google Scholar]
- 4. Virani SS, Maddox TM, Chan PS, et al. Provider type and quality of outpatient cardiovascular disease care: insights from the NCDR PINNACLE Registry. J Am Coll Cardiol. 2015;66:1803–1812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Rodgers GP, Conti JB, Feinstein JA, et al. ACC 2009 survey results and recommendations: addressing the cardiology workforce crisis: a report of the ACC Board of Trustees Workforce Task Force. J Am Coll Cardiol. 2009;54:1195–1208. [DOI] [PubMed] [Google Scholar]
- 6. Brush JE Jr, Handberg EM, Biga C, et al. 2015 ACC Health Policy Statement on cardiovascular team‐based care and the role of advanced practice providers. J Am Coll Cardiol. 2015;65:2118–2136. [DOI] [PubMed] [Google Scholar]
- 7. Dunn SP, Birtcher KK, Beavers CJ, et al. The role of the clinical pharmacist in the care of patients with cardiovascular disease. J Am Coll Cardiol. 2015;66:2129–2139. [DOI] [PubMed] [Google Scholar]
- 8. Virani SS, Akeroyd JM, Ramsey DJ, et al. Comparative effectiveness of outpatient cardiovascular disease and diabetes care delivery between advanced practice providers and physician providers in primary care: implications for care under the Affordable Care Act. Am Heart J. 2016;181:74–82. [DOI] [PubMed] [Google Scholar]
- 9. Jessup M, Albert NM, Lanfear DE, et al. ACCF/AHA/HFSA 2011 survey results: current staffing profile of heart failure programs, including programs that perform heart transplant and mechanical circulatory support device implantation: a report of the ACCF Heart Failure and Transplant Committee, AHA Heart Failure and Transplantation Committee, and Heart Failure Society of America. J Am Coll Cardiol. 2011;57:2115–2124. [DOI] [PubMed] [Google Scholar]
- 10. Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection , Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) final report. Circulation. 2002;106:3143–3421. [PubMed] [Google Scholar]
- 11. Grundy SM, Cleeman JI, Merz CN, et al; National Heart, Lung, and Blood Institute; American College of Cardiology Foundation; American Heart Association . Implications of recent clinical trials for the National Cholesterol Education Program Adult Treatment Panel III Guidelines [published correction appears in Circulation 2004;110:763]. Circulation. 2004;110:227–239. [DOI] [PubMed] [Google Scholar]
- 12. D'Agostino RB, Vasan RS, Pencina MJ, et al. General cardiovascular risk profile for use in primary care: the Framingham Heart Study. Circulation. 2008;117:743–753. [DOI] [PubMed] [Google Scholar]
- 13. Agatston AS, Janowitz WR, Hildner FJ, et al. Quantification of coronary artery calcium using ultrafast computed tomography. J Am Coll Cardiol. 1990;15:827–832. [DOI] [PubMed] [Google Scholar]
- 14. Fonarow GC, Gawlinski A. Rationale and design of the Cardiac Hospitalization Atherosclerosis Management Program at the University of California Los Angeles. Am J Cardiol. 2000;85:10A–17A. [DOI] [PubMed] [Google Scholar]
- 15. Olson KL, Rasmussen J, Sandhoff BG, et al; Clinical Pharmacy Cardiac Risk Service Study Group . Lipid management in patients with coronary artery disease by a clinical pharmacy service in a group model health maintenance organization. Arch Intern Med. 2005;165:49–54. [DOI] [PubMed] [Google Scholar]
- 16. Todd BA, Lamprecht DG Jr, Stadler SL. Pharmacist prescribing practices in a clinical pharmacy cardiac risk service. Am J Health Sys Pharm. 2016;73:1442–1450. [DOI] [PubMed] [Google Scholar]
- 17. Sandhoff BG, Kuca S, Rasmussen J, et al. Collaborative cardiac care service: a multidisciplinary approach to caring for patients with coronary artery disease. Perm J. 2008;12:4–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Pape GA, Hunt JS, Butler KL, et al. Team‐based care approach to cholesterol management in diabetes mellitus: two‐year cluster randomized controlled trial. Arch Intern Med. 2011;171:1480–1486. [DOI] [PubMed] [Google Scholar]
- 19. Isetts BJ, Buffington DE, Carter BL, et al. Evaluation of pharmacists' work in a physician‐pharmacist collaborative model for the management of hypertension. Pharmacotherapy. 2016;36:374–384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ferket BS, Hunink MG, Khanji M, et al. Cost‐effectiveness of the polypill versus risk assessment for prevention of cardiovascular disease. Heart. 2017;103:483–491. [DOI] [PubMed] [Google Scholar]
- 21. Traczynski J, Udalova V. Nurse practitioner independence, health care utilization, and health outcomes. J Health Econ. 2018;58:90–109. [DOI] [PubMed] [Google Scholar]
- 22. Mehta S, Ghosh S, Sander S, et al. Differences in all‐cause health care utilization and costs in a type 2 diabetes mellitus population with and without a history of cardiovascular disease. J Manag Care Spec Pharm. 2018;24:280–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. DeFilippis AP, Blaha MJ, Ndumele CE, et al. The association of Framingham and Reynolds risk scores with incidence and progression of coronary artery calcification in MESA (Multi‐Ethnic Study of Atherosclerosis). J Am Coll Cardiol. 2011;58:2076–2083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Melander O, Newton‐Cheh C, Almgren P, et al. Novel and conventional biomarkers for prediction of incident cardiovascular events in the community. JAMA. 2009;302:49–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Erbel R, Möhlenkamp S, Moebus S, et al; Heinz Nixdorf Recall Study Investigative Group . Coronary risk stratification, discrimination, and reclassification improvement based on quantification of subclinical coronary atherosclerosis: the Heinz Nixdorf Recall study. J Am Coll Cardiol. 2010;56:1397–1406. [DOI] [PubMed] [Google Scholar]
- 26. Yeboah J, Polonsky TS, Young R, et al. Utility of nontraditional risk markers in individuals ineligible for statin therapy according to the 2013 American College of Cardiology/American Heart Association Cholesterol Guidelines. Circulation. 2015;132:916–922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Nasir K, Bittencourt MS, Blaha MJ, et al. Implications of coronary artery calcium testing among statin candidates according to American College of Cardiology/American Heart Association Cholesterol Management Guidelines: MESA (Multi‐Ethnic Study of Atherosclerosis). J Am Coll Cardiol. 2015;66:1657–1668. [DOI] [PubMed] [Google Scholar]
- 28. Rozanski A, Gransar H, Shaw LJ, et al. Impact of coronary artery calcium scanning on coronary risk factors and downstream testing: the EISNER (Early Identification of Subclinical Atherosclerosis by Noninvasive Imaging Research) prospective randomized trial. J Am Coll Cardiol. 2011;57:1622–1632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Gupta A, Lau E, Varshney R, et al. The identification of calcified coronary plaque is associated with initiation and continuation of pharmacological and lifestyle preventive therapies: a systematic review and meta‐analysis. JACC Cardiovasc Imaging. 2017;10:833–842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Cavanaugh‐Hussey MW, Berry JD, Lloyd‐Jones DM. Who exceeds ATP‐III risk thresholds? Systematic examination of the effect of varying age and risk factor levels in the ATP‐III risk assessment tool. Prev Med. 2008;47:619–623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Marma AK, Lloyd‐Jones DM. Systematic examination of the updated Framingham heart study general cardiovascular risk profile. Circulation. 2009;120:384–390. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Distribution of Coronary Artery Calcium Scores (CACS) Among PCC patients (A) 10‐ year FRS predicted ASCVD risk among different CAC score groups (B) CACS distribution amongst risk groups. (C) MESA percentile distribution amongst patients with and without CACS >100 arbitrary units.
Figure S2. Lipid Lowering Therapies and Statin Intensity per FRS category Initial and at follow‐up post‐intervention amongst (A) PMC patients and (B) PCC patients. Panel C shows post‐intervention lipid therapy treatment data in treatment naïve patients in the PMC and PCC cohorts. Panel D depicts overall lipid therapy changes amongst PCC and


