Abstract
Background
Pulmonary embolism (PE) is associated with significant morbidity and mortality in hospitalized patients. Real time data on 90‐day mortality, bleeding, and readmission is sparse.
Methods
The study cohort was derived from the National Readmission Data (NRD) 2013 to 2014. PE was identified using International Classification of Diseases, ninth Revision (ICD‐9‐CM) code 415.11/3/9 in the primary diagnosis field. Any admission within 90 days of primary admission was considered a 90‐day readmission. Readmission etiologies were identified by ICD‐9 code in the primary diagnosis field. Co‐primary outcomes were 90‐day readmission and 90‐day mortality.
Results
We identified 260 614 patients with primary admission PE, 55 659 (21.36%) patients were readmitted within 90 days. Most of them were of old age (age ≥ 65 years: 49.04%) and females (52.78%). Among the etiologies of readmission pulmonary disorders (22.94%) (Including recurrent PE 7.33%), malignancies (8.31%), and bleeding disorders (6.75%) were the most important causes of 90‐day readmissions. On multivariate analysis, higher readmission rates and 90 days mortality were seen in patients with heart failure, chronic pulmonary disease, Anemia, malignancy, and with higher Charlson score. Patients with longer length of stay during primary admission and who discharged to short/long‐term facility were more likely get readmitted and die in 90 days. Paradoxically, obese patients showed an inverse relationship with co‐primary outcomes.
Conclusions
Older female patients were more likely to have a pulmonary embolism. High‐risk groups such as heart failure, chronic pulmonary disease, anemia, and malignancy need to be given extra attention to prevent worse outcomes.
Keywords: comorbidities, mortality, outcomes, pulmonary embolism, readmission
1. INTRODUCTION
Pulmonary embolism (PE) is associated with significant morbidity and mortality in hospitalized patients. The incidence of pulmonary embolism was nearly 69 cases per 100 000 persons in 1998.1 With increased recognition of PE using the more sensitive computed tomography pulmonary angiogram in 1998, the incidence of PE has increased to 112.3 cases per 100 000 persons by year 2011.2 Although the financial burden associated with PE alone is not known, venous thromboembolism (VTE) has been known to cost the US healthcare system nearly $500 million dollars per year,3 with an estimated mean cost for a PE hospitalization of $16 644 and mean cost of readmission of $14 722.3, 4
The American College of Chest Physicians recommends anticoagulation for 3 months after a proximal DVT or PE that is provoked by a surgery or a transient non‐surgical risk factor. For unprovoked DVT or PE, they recommend anticoagulation for 3 months if low risk of bleeding.5 Treatment of PE with anticoagulation is also associated with the risk of bleeding. Limited real time data exist in the current literature regarding the short‐term outcomes of PE patients. We decided to analyze outcomes including readmission, mortality, and bleeding over 90 days.
2. METHODS
2.1. Data source
The study cohort was derived from the National Readmission Database (NRD) of 2013 and 2014, a subset of the Healthcare Cost and Utilization Project (HCUP) sponsored by the Agency for Healthcare Research and Quality (AHRQ). The NRD is one of the largest publicly available all‐payer inpatient care database in the United States. Unweighted, the NRD contains data from approximately 15 million discharges each year and weighted, it estimates roughly 35 million discharges in the United States. NRD represents 49.1% of total US hospitalizations. Patients were tracked using variable “NRD_visitlink” and time between two admissions was calculated by subtracting variable “NRD_DaysToEvent.” Time to readmission was calculated by subtracting length of stay of index admissions to time between two admissions. The details regarding the NRD data are available online.6
2.2. Data
Pulmonary embolism hospitalizations were classified as those with a principal discharge diagnosis of Pulmonary Embolism based on the International Classification of Diseases, ninth revision, Clinical Modification (ICD 9‐CM) code 415.11/3/9. We only included first admission of the year and excluded patients with missing information on age, sex, mortality, and age ≤ 18. We also excluded October, November, and December admissions, as we did not have follow‐up data on them. We identified total of 260 614 primary admissions. Patients who were readmitted to the hospital within 90 days during same calendar year were further evaluated (n = 55 659). (Figure S1, Supporting information).
2.3. Outcomes
Co‐primary outcomes were 90‐day readmission and 90‐day mortality. Secondary outcome was combination of 90‐day mortality, gastrointestinal bleeding, and intracranial hemorrhage. The causes of readmissions were identified using ICD‐9 codes in primary diagnosis field. We identified 2064 ICD 9 diagnosis codes and combined the ones with similar diagnoses to make clinically important groups, which were verified by two co‐authors (Purav Shah and Varun Kumar) (Table S1). We also did subgroup analysis for surgical PEs. PE patients with surgical DRG codes in past 90 days (previous hospitalization) were identified as surgical PEs.
2.4. Demographics, comorbidities, and other independent variables
NRD variables were used to identify patients' demographic characteristics including age; gender; hospital characteristics such as bed size and teaching status; other patient specific characteristics including median household income category for patient's zip code, primary payer, admission type, admission day, and discharge disposition.6 Comorbid variables provided by HCUP indicate different comorbidities by using ICD‐9‐CM diagnoses and the diagnosis‐related group in effect on the discharge date.7 These comorbidities are not directly related to the principal diagnosis or the main reason for admission and are likely to have originated before the hospital stay. Severity of comorbid conditions defined using Deyo modification of Charlson comorbidity index (CCI), which contains 17 comorbid conditions with differential weights. The score ranges from 0 to 33, with higher scores corresponding to greater burden of comorbid diseases8 (Table S2).
2.5. Statistical software and methods
SAS 9.4 (SAS Institute Inc., Cary, North Carolina) was utilized for analyses. Differences between categorical variables were tested using the χ 2 test and differences between continuous variables were tested using student's t test. We used cox proportional regression to adjust for confounder and consider timing in consideration too. We ran Kaplan Meyer curve for every outcome before running final multivariate models (Figures [Link], [Link], [Link]). In multivariate models for readmission, 90 day mortality and 90 day mortality/ICH/GI bleed, we included patient level variables like age, sex, primary payer [Medicare/Medicaid as reference, private insurance including HMO, self‐pay]; comorbidities like obesity, diabetes, peripheral vascular disease, renal failure, anemia; disposition during principal admission (facility vs home), median household income (0‐25 percentile as reference, 26‐50 percentile, 51‐75 percentile, 76‐100 percentile); length of stay (≤2 as reference, 3‐4, 5‐6, >6) and hospital characteristics such as hospital bed size(small as reference, medium, large), hospital teaching status (teaching vs non‐teaching). Multivariate model for readmission was run only on patients who survived index admission. We also ran post hoc analysis of 30‐day readmission for validation of our results (Table S3).
3. RESULTS
We identified 260, 614 patients with primary admission of pulmonary embolism in year 2013‐14, out of which 55, 659(21.36%) patients were readmitted within 90 days post discharge. Cohort consists of mainly old age (age ≥ 65 years) and female (52.78%) patients. Most common comorbidities were hypertension (58.02%), chronic pulmonary disease (24.79%), diabetes (21.97%), and obesity (21.75%). Majority of the patients were admitted to large hospitals (57.46%), teaching hospitals (53.03%), during weekdays (77.5%), and covered Medicaid/Medicare (61.79%). Average length of stay during primary admission was 5.09 days with 13.6% patients discharged to short/long term facility and 3.12% died during primary admission only (Table 1).
Table 1.
Baseline characteristics of overall pulmonary embolism and surgical pulmonary embolism
| Overall pulmonary embolism | Surgical pulmonary embolism | |||||||
|---|---|---|---|---|---|---|---|---|
| Variables | Readmission | Readmission | ||||||
| No | Yes | Overall | P value | No | Yes | Overall | P value | |
| Unweighted index admission | 89 143(78.6%) | 24 339(21.5%) | 113 482 | 10 365(73.1%) | 3819(26.9%) | 14 184 | ||
| Weighted index admission | 204 955(78.6%) | 55 659(21.4%) | 260 614 | 22 472 (72.9%) | 8371 (27.4%) | 30 844 | ||
| Patient level variables | ||||||||
| Age | <0.001 | 0.5321 | ||||||
| 18‐34 | 7.57 | 5.55 | 7.14 | 4.93 | 5.07 | 4.97 | ||
| 35‐49 | 16.24 | 13.95 | 15.76 | 11.94 | 12.19 | 12.01 | ||
| 50‐64 | 28.06 | 28.06 | 28.06 | 29.44 | 30.17 | 29.64 | ||
| 65‐79 | 30.85 | 32.82 | 31.27 | 37.81 | 37.07 | 37.61 | ||
| ≥80 | 17.28 | 19.61 | 17.77 | 15.89 | 15.5 | 15.78 | ||
| Gender | <0.001 | 0.7899 | ||||||
| Male | 47.73 | 45.35 | 47.22 | 46.01 | 46.18 | 46.06 | ||
| Female | 52.27 | 54.65 | 52.78 | 53.99 | 53.82 | 53.94 | ||
| Deyo/Charlson score a | <0.001 | <0.001 | ||||||
| 0 | 43.81 | 23.28 | 39.42 | 39.72 | 22.33 | 35 | ||
| 1 | 24.13 | 22.76 | 23.84 | 22.75 | 20.83 | 22.23 | ||
| ≥2 | 32.06 | 53.96 | 36.74 | 37.53 | 56.84 | 42.77 | ||
| Comorbidities | ||||||||
| Obesityb | 22.16 | 20.26 | 21.75 | <0.001 | 19.96 | 19.23 | 19.76 | 0.1499 |
| History of hypertensionb | 56.77 | 62.59 | 58.02 | <0.001 | 61.41 | 65.49 | 62.52 | <0.001 |
| History of diabetesb | 20.6 | 27.02 | 21.97 | <0.001 | 21.33 | 28.69 | 23.33 | <0.001 |
| Heart failuref | 11.66 | 20.3 | 13.5 | <0.001 | 11.39 | 18.96 | 13.44 | <0.001 |
| History of chronic pulmonary diseaseb | 22.5 | 33.23 | 24.79 | <0.001 | 21.3 | 28.59 | 23.28 | <0.001 |
| Peripheral vascular diseaseb | 4.95 | 6.72 | 5.33 | <0.001 | 6.06 | 9.3 | 6.94 | <0.001 |
| Renal failure | 9.47 | 14.34 | 10.51 | <0.001 | 9.54 | 15.29 | 11.1 | <0.001 |
| Neurological disorder or paralysis | 8.81 | 11.94 | 9.48 | <0.001 | 8.72 | 11.22 | 9.4 | <0.001 |
| Anemiab | 16.6 | 25.97 | 18.6 | <0.001 | 26 | 32.74 | 27.83 | <0.001 |
| Hematological or oncological malignancyb | 11.55 | 23.41 | 14.08 | <0.001 | 17.99 | 28.33 | 20.79 | <0.001 |
| Rheumatoid arthritis or another collagen vascularb | 3.48 | 4.61 | 3.72 | <0.001 | 3.06 | 4.69 | 3.5 | <0.001 |
| Depression, psychosis or substance abuseb | 19.58 | 24.96 | 20.73 | <0.001 | 16.45 | 21.13 | 17.72 | <0.001 |
| Median household income category for patient's zip code c | <0.001 | <0.001 | ||||||
| 0‐25th percentile | 25.05 | 29.74 | 26.05 | 24.9 | 28.58 | 25.9 | ||
| 26‐50th percentile | 27.25 | 27.27 | 27.25 | 26.55 | 26.64 | 26.58 | ||
| 51‐75th percentile | 24.35 | 22.74 | 24.01 | 24.93 | 23.08 | 24.43 | ||
| 76‐100th percentile | 21.72 | 18.84 | 21.1 | 22.11 | 20.65 | 21.72 | ||
| Primary payer | <0.001 | <0.001 | ||||||
| Medicare/Medicaid | 59.02 | 72 | 61.79 | 63.06 | 70.75 | 65.14 | ||
| Private including HMO | 32.36 | 20.92 | 29.92 | 31.03 | 25.03 | 29.4 | ||
| Self‐pay/no charge/other | 8.52 | 6.95 | 8.18 | 5.79 | 4.11 | 5.33 | ||
| Hospital characteristics | ||||||||
| Hospital bed size d | <0.001 | <0.001 | ||||||
| Small | 16.21 | 13.78 | 15.69 | 15.44 | 12.54 | 14.65 | ||
| Medium | 27.15 | 25.72 | 26.84 | 26.54 | 24.6 | 26.02 | ||
| Large | 56.64 | 60.49 | 57.46 | 58.02 | 62.86 | 59.33 | ||
| Hospital teaching status e | <0.001 | |||||||
| Non‐teaching | 47.74 | 44.09 | 46.97 | 41.05 | 37.22 | 40.01 | ||
| Teaching | 52.26 | 55.91 | 53.03 | 58.95 | 62.78 | 59.99 | ||
| Admission day | 0.6491 | 0.996 | ||||||
| Weekdays | 77.48 | 77.57 | 77.5 | 77.26 | 77.26 | 77.26 | ||
| Weekend | 22.52 | 22.43 | 22.5 | 22.74 | 22.74 | 22.74 | ||
| Disposition | <0.001 | |||||||
| Home | 83.76 | 78.31 | 82.6 | 77.23 | 73.78 | 76.29 | ||
| Facility | 11.75 | 20.39 | 13.6 | 18.08 | 25.32 | 20.05 | ||
| In hospital mortality | 3.97 | 0 | 3.12 | 4.37 | 0 | 3.18 | ||
| Length of stay | 4.82 ± 0.02 | 6.08 ± 0.04 | 5.09 ± 0.02 | <0.001 | 5.04 ± 0.05 | 6.08 ± 0.09 | 5.35 ± 0.05 | <0.001 |
| Transfusion | 3.96 | 7.38 | 4.69 | <0.001 | 5.93 | 9.21 | 6.82 | <0.001 |
| Cardiac arrest | 1.65 | 0.54 | 1.41 | <0.001 | 1.73 | 0.6 | 1.43 | <0.001 |
| Cardiogenic shock | 0.95 | 0.52 | 0.85 | <0.001 | 1.23 | 0.64 | 1.07 | <0.001 |
| Use of vasopressor | 0.45 | 0.29 | 0.41 | <0.001 | 0.03 | 0.02 | 0.02 | 0.7672 |
| Intracranial hemorrhage (ICH) | 0.29 | 0.25 | 0.29 | 0.1096 | 0.55 | 0.7 | 0.59 | 0.142 |
| Gastrointestinal bleed (GI bleed) | 1.5 | 2.18 | 1.64 | <0.001 | 1.51 | 2.51 | 1.78 | <0.001 |
| Other bleeding complications | 0.47 | 0.64 | 0.51 | <0.001 | 1.28 | 1.23 | 1.26 | 0.7306 |
| Any bleed | 2.23 | 2.99 | 2.39 | <0.001 | 3.26 | 4.35 | 3.56 | <0.001 |
| Hemorrhage requiring transfusion | 0.64 | 1.03 | 0.73 | <0.001 | 0.98 | 1.5 | 1.12 | <0.001 |
Abbreviation: HMO, Health Maintenance Organization.
Charlson/Deyo comorbidity index (CCI) was calculated as per Deyo classification.
Variables are AHRQ comorbidity measures.
Represents a quartile classification of the estimated median household income of residents in the patients ZIP code, derived from ZIP code‐demographic data obtained from Claritas. The quartiles are identified by values of 1 to 4, indicating the poorest to wealthiest populations. Because these estimates are updated annually, the value ranges vary by year. https://www.hcup-us.ahrq.gov/db/vars/zipinc_qrtl/nrdnote.jsp.
The bed size cutoff points divided into small, medium, and large have been done so that approximately one‐third of the hospitals in a given region, location, and teaching status combination would fall within each bed size category. https://www.hcup-us.ahrq.gov/db/vars/hosp_bedsize/nrdnote.jsp.
A hospital is considered to be a teaching hospital if it has an AMA‐approved residency program, is a member of the Council of Teaching Hospitals (COTH) or has a ratio of full‐time equivalent interns and residents to beds of 0.25 or higher. https://www.hcup-us.ahrq.gov/db/vars/hosp_ur_teach/nrdnote.jsp.
Comorbidity was identified by appropriate ICD 9 CM diagnosis codes in secondary diagnosis field.
Pulmonary disorders (22.94%) were the most common causes of readmission. About 7.33% of these readmissions were secondary to recurrent PE. Malignancy contributed 8.31%, and bleeding was associated with 6.75% of the 90‐day readmissions. About 3.68% patients were readmitted due to a gastrointestinal bleeding and 0.82% patients had an intracranial bleed. About 2.25% patients were admitted due to a non‐gastrointestinal or a non‐intracranial bleed. Cardiovascular disorder (12.81%), non‐respiratory tract infections (10.48%), and neurologic disorders (4%) were the other significant etiologies of readmissions (Table 2) (Figure 1).
Table 2.
Etiologies of readmissions among overall pulmonary embolism
| Etiology | Value (%) |
|---|---|
| Pulmonary disorders | 22.94 |
| Asthma | 0.76 |
| COPD exacerbation and related disorders | 2.60 |
| Respiratory failure | 2.43 |
| Respiratory infections | 5.12 |
| Pulmonary embolism | 7.33 |
| Others | 4.70 |
| Infections | 10.48 |
| Neoplasms | 8.31 |
| Solid | 7.49 |
| Liquid | 0.82 |
| Hematological disorders | 3.58 |
| Endocrine disorders | 1.10 |
| Rheumatic/autoimmune/musculoskeletal | 2.45 |
| Kidney and urinary tract disorders | 4.97 |
| Electrolyte and Acid‐Base disorders | 1.47 |
| Neurologic/psychiatric disorders | 6.67 |
| Neurological | 4.00 |
| Psychiatric | 2.67 |
| Cardiovascular disorders | 12.81 |
| Heart failure | 5.08 |
| CAD/IHD | 1.61 |
| Rhythm disorder | 2.38 |
| Valvular disorder | 0.23 |
| Hypertension and related complications | 0.71 |
| Others | 2.80 |
| Other vascular disorders | 3.62 |
| Gastrointestinal disorders | 7.00 |
| Trauma/fracture/poisoning | 1.69 |
| Others | 3.58 |
| Bleeding | 6.75 |
| Intracranial hemorrhage | 0.82 |
| GI bleed | 3.68 |
| Any bleed | 2.25 |
Abbreviations: CAD, coronary artery disease; IHD, ischemic heart disease.
Figure 1.
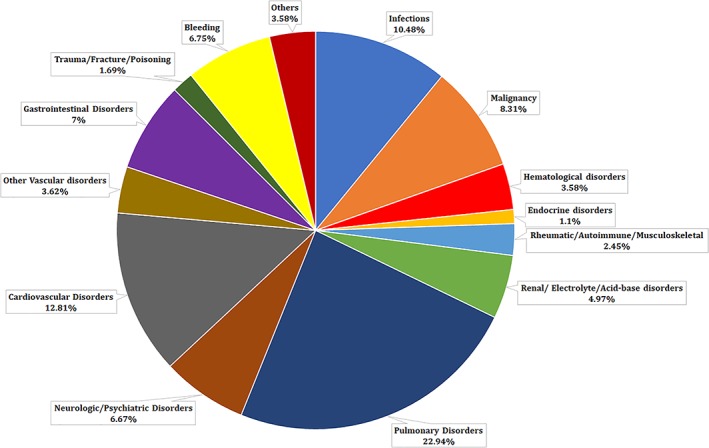
Etiologies of readmissions with overall pulmonary embolism
In the multivariate analysis, malignancies were associated with the highest readmission rates (HR 1.63, CI 1.56‐1.70, P < 0.001), followed by longer length of stay (LOS <2 days reference; LOS >6 days HR 1.47, CI 1.42‐1.53, P < 0.001), higher Charlson/Deyo comorbidity score (score 1 HR 1.40, CI 1.34‐1.46, P < 0.001; score ≥ 2 HR 1.78, CI 1.68‐1.87, P < 0.001), heart failure (HR 1.30, CI 1.25‐1.35, P < 0.001), anemia (HR 1.27, CI 1.23‐1.31, P < 0.001), and neurologic disorders and paralysis (HR 1.17, CI 1.12‐1.22, P < 0.001). Females had a slightly higher risk of readmission compared to males (HR 1.03, CI 1.01 1.06, P = 0.012), and obesity was associated with lower readmission rates (HR 0.86, CI 0.83‐0.89, P < 0.001) (Table 3). Post hoc analysis of 30‐day readmission showed similar results (Table S3).
Table 3.
Multivariate analysis of 90‐day and 30‐day readmission of overall pulmonary embolism and surgical pulmonary embolism
| Overall pulmonary embolism | Surgical pulmonary embolism | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Variables | 90‐day readmission | 30‐day readmission | 90‐day readmission | 30‐day readmission | ||||||||||||
| HR | LL | UL | P value | HR | LL | UL | P value | HR | LL | UL | P value | HR | LL | UL | P value | |
| Age | 0.99 | 0.99 | 0.99 | <0.0001 | 0.99 | 0.99 | 0.99 | <0.0001 | 0.99 | 0.99 | 0.99 | <0.0001 | 0.99 | 0.99 | 0.99 | <0.0001 |
| Gender | ||||||||||||||||
| Male | Ref | Ref | Ref | Ref | Ref | Ref | Ref | Ref | Ref | Ref | Ref | Ref | ||||
| Female | 1.03 | 1.01 | 1.06 | 0.012 | 1.04 | 1.01 | 1.08 | 0.018 | 0.98 | 0.92 | 1.05 | 0.620 | 0.97 | 0.90 | 1.06 | 0.523 |
| Deyo/Charlson score a | ||||||||||||||||
| 0 | Ref | Ref | Ref | Ref | Ref | Ref | Ref | Ref | Ref | Ref | Ref | Ref | ||||
| 1 | 1.40 | 1.34 | 1.46 | <0.0001 | 1.40 | 1.32 | 1.48 | <0.0001 | 1.33 | 1.20 | 1.49 | <0.0001 | 1.31 | 1.14 | 1.50 | 0.000 |
| ≥2 | 1.78 | 1.68 | 1.87 | <0.0001 | 1.79 | 1.67 | 1.92 | <0.0001 | 1.59 | 1.40 | 1.82 | <0.0001 | 1.51 | 1.27 | 1.78 | <0.0001 |
| Comorbidities | ||||||||||||||||
| Obesityb | 0.86 | 0.83 | 0.89 | <0.0001 | 0.85 | 0.81 | 0.88 | <0.0001 | 0.89 | 0.81 | 0.97 | 0.006 | 0.89 | 0.80 | 0.99 | 0.039 |
| History of hypertensionb | 1.05 | 1.02 | 1.08 | 0.001 | 1.04 | 1.00 | 1.08 | 0.029 | 1.06 | 0.98 | 1.14 | 0.160 | 1.06 | 0.96 | 1.16 | 0.248 |
| History of diabetesb | 1.05 | 1.02 | 1.09 | 0.004 | 1.08 | 1.03 | 1.13 | 0.001 | 1.14 | 1.05 | 1.24 | 0.002 | 1.13 | 1.02 | 1.25 | 0.025 |
| Heart failuref | 1.30 | 1.25 | 1.35 | <0.0001 | 1.33 | 1.27 | 1.39 | <0.0001 | 1.34 | 1.22 | 1.47 | <0.0001 | 1.33 | 1.19 | 1.50 | <0.0001 |
| History of chronic pulmonary diseaseb | 1.16 | 1.13 | 1.20 | <0.0001 | 1.19 | 1.14 | 1.24 | <0.0001 | 1.11 | 1.02 | 1.20 | 0.013 | 1.18 | 1.06 | 1.30 | 0.001 |
| Peripheral vascular diseaseb | 1.07 | 1.02 | 1.13 | 0.007 | 1.08 | 1.01 | 1.15 | 0.025 | 1.22 | 1.09 | 1.36 | 0.001 | 1.15 | 1.00 | 1.33 | 0.057 |
| Renal failure | 1.02 | 0.97 | 1.06 | 0.458 | 1.05 | 0.99 | 1.10 | 0.122 | 1.21 | 1.09 | 1.34 | 0.001 | 1.18 | 1.03 | 1.35 | 0.016 |
| Neurological disorder or paralysis | 1.17 | 1.12 | 1.22 | <0.0001 | 1.17 | 1.11 | 1.23 | <0.0001 | 1.09 | 0.98 | 1.21 | 0.120 | 1.10 | 0.96 | 1.25 | 0.186 |
| Anemiab | 1.27 | 1.23 | 1.31 | <0.0001 | 1.31 | 1.26 | 1.36 | <0.0001 | 1.12 | 1.04 | 1.20 | 0.002 | 1.14 | 1.05 | 1.25 | 0.003 |
| Hematological or oncological malignancyb | 1.63 | 1.56 | 1.70 | <0.0001 | 1.76 | 1.66 | 1.85 | <0.0001 | 1.47 | 1.33 | 1.64 | <0.0001 | 1.60 | 1.40 | 1.82 | <0.0001 |
| Rheumatoid arthritis or another collagen vascularb | 1.06 | 1.00 | 1.13 | 0.060 | 1.02 | 0.94 | 1.11 | 0.600 | 1.15 | 0.98 | 1.36 | 0.092 | 1.11 | 0.90 | 1.36 | 0.341 |
| Depression, psychosis or substance abuseb | 1.20 | 1.16 | 1.23 | <0.0001 | 1.20 | 1.15 | 1.25 | <0.0001 | 1.20 | 1.11 | 1.31 | <0.0001 | 1.16 | 1.04 | 1.28 | 0.006 |
| Median household income category for patient's zip code c | ||||||||||||||||
| 0‐25th percentile | Ref | Ref | Ref | Ref | Ref | Ref | Ref | Ref | Ref | Ref | Ref | Ref | ||||
| 26‐50th percentile | 0.94 | 0.91 | 0.98 | 0.001 | 0.94 | 0.90 | 0.98 | 0.006 | 0.94 | 0.86 | 1.02 | 0.155 | 0.93 | 0.83 | 1.04 | 0.210 |
| 51‐75th percentile | 0.94 | 0.90 | 0.97 | <0.0001 | 0.94 | 0.90 | 0.98 | 0.006 | 0.93 | 0.85 | 1.02 | 0.118 | 0.91 | 0.81 | 1.02 | 0.109 |
| 76‐100th percentile | 0.90 | 0.87 | 0.94 | <0.0001 | 0.92 | 0.88 | 0.97 | 0.001 | 0.94 | 0.85 | 1.03 | 0.185 | 0.99 | 0.88 | 1.11 | 0.801 |
| Primary payer | ||||||||||||||||
| Medicare/Medicaid | Ref | Ref | Ref | Ref | Ref | Ref | Ref | Ref | Ref | Ref | Ref | Ref | ||||
| Private including HMO | 0.68 | 0.66 | 0.71 | <0.0001 | 0.68 | 0.65 | 0.71 | <0.0001 | 0.78 | 0.71 | 0.84 | <0.0001 | 0.80 | 0.72 | 0.89 | <0.0001 |
| Self‐pay/no charge/other | 0.81 | 0.76 | 0.85 | <0.0001 | 0.81 | 0.75 | 0.87 | <0.0001 | 0.75 | 0.64 | 0.88 | 0.000 | 0.83 | 0.68 | 1.01 | 0.061 |
| Hospital characteristics | ||||||||||||||||
| Hospital bed size d | ||||||||||||||||
| Small | Ref | Ref | Ref | Ref | Ref | Ref | Ref | Ref | Ref | Ref | Ref | Ref | ||||
| Medium | 1.08 | 1.03 | 1.12 | 0.001 | 1.08 | 1.02 | 1.14 | 0.011 | 1.09 | 0.97 | 1.22 | 0.135 | 1.04 | 0.90 | 1.20 | 0.630 |
| Large | 1.11 | 1.07 | 1.16 | <0.0001 | 1.12 | 1.06 | 1.18 | <0.0001 | 1.17 | 1.06 | 1.30 | 0.003 | 1.12 | 0.98 | 1.27 | 0.092 |
| Hospital teaching status e | ||||||||||||||||
| Non‐teaching | Ref | Ref | Ref | Ref | Ref | Ref | Ref | Ref | Ref | Ref | Ref | Ref | ||||
| Teaching | 1.08 | 1.05 | 1.11 | <0.0001 | 1.09 | 1.06 | 1.13 | <0.0001 | 1.05 | 0.98 | 1.12 | 0.151 | 1.03 | 0.95 | 1.12 | 0.494 |
| Disposition | ||||||||||||||||
| Home | Ref | Ref | Ref | Ref | Ref | Ref | Ref | Ref | Ref | Ref | Ref | Ref | ||||
| Facility | 1.24 | 1.19 | 1.28 | <0.0001 | 1.24 | 1.19 | 1.30 | <0.0001 | 1.16 | 1.07 | 1.25 | 0.001 | 1.08 | 0.97 | 1.20 | 0.159 |
| Length of stay | ||||||||||||||||
| ≤2 | Ref | Ref | Ref | Ref | Ref | Ref | Ref | Ref | Ref | Ref | Ref | Ref | ||||
| 3‐6 | 1.21 | 1.16 | 1.25 | <0.0001 | 1.22 | 1.17 | 1.27 | <0.0001 | 1.12 | 1.03 | 1.22 | 0.011 | 1.17 | 1.05 | 1.31 | 0.004 |
| >6 | 1.47 | 1.42 | 1.53 | <0.0001 | 1.53 | 1.46 | 1.61 | <0.0001 | 1.38 | 1.26 | 1.52 | <0.0001 | 1.44 | 1.27 | 1.62 | <0.0001 |
Abbreviation: HMO, Health Maintenance Organization.
Charlson/Deyo comorbidity index (CCI) was calculated as per Deyo classification.
Variables are AHRQ comorbidity measures.
Represents a quartile classification of the estimated median household income of residents in the patients ZIP code, derived from ZIP code‐demographic data obtained from Claritas. The quartiles are identified by values of 1 to 4, indicating the poorest to wealthiest populations. Because these estimates are updated annually, the value ranges vary by year. https://www.hcup-us.ahrq.gov/db/vars/zipinc_qrtl/nrdnote.jsp.
The bed size cutoff points divided into small, medium, and large have been done so that approximately one‐third of the hospitals in a given region, location, and teaching status combination would fall within each bed size category. https://www.hcup-us.ahrq.gov/db/vars/hosp_bedsize/nrdnote.jsp.
A hospital is considered to be a teaching hospital if it has an AMA‐approved residency program, is a member of the Council of Teaching Hospitals (COTH) or has a ratio of full‐time equivalent interns and residents to beds of 0.25 or higher. https://www.hcup-us.ahrq.gov/db/vars/hosp_ur_teach/nrdnote.jsp.
Comorbidity was identified by appropriate ICD 9 CM diagnosis codes in secondary diagnosis field.
The 90‐day mortality rate was 4.92% (12 820 out of the 260 614 patients died) (Figure S2). Ninety‐day mortality rates were highest in patients with underlying malignancies (HR 3.29, CI 2.91‐3.73, P < 0.001) and a Charlson/Deyo comorbidity index ≥2 (HR 3.38, CI 2.76‐4.13, P < 0.001). Mortality rates were also higher in patients with heart failure (HR: 1.35, CI: 1.21‐1.51, P < 0.001), chronic pulmonary disease (HR: 1.16, 1.05‐1.28, p‐0.003), and anemia (HR 1.30, CI 1.19‐1.44, P < 0.001). Compared to Medicare/Medicaid, lower mortality rates were seen in patients with private insurance and HMOs (HR 0.94, CI 0.83‐1.06, P = 0.311) and self‐paid/other insurance (HR 0.77, CI 0.61‐0.98, P = 0.03). Longer length of stay and discharge to short/long term facility were associated with higher 90‐day mortality (Table 4). Similar results were observed for secondary outcomes (Table 4).
Table 4.
Multivariate of 90‐day mortality and 90‐day mortality and bleeding among overall pulmonary embolism and surgical pulmonary embolism
| Overall pulmonary embolism | Surgical pulmonary embolism | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Variables | 90‐day mortality | 90‐day mortality/GI bleed/ICH | 90‐day mortality | 90‐day mortality/GI bleed/ICH | ||||||||||||
| HR | LL | UL | P value | HR | LL | UL | P‐value | HR | LL | UL | P‐value | HR | LL | UL | P‐value | |
| Age | 1.01 | 1.00 | 1.01 | 0.001 | 1.01 | 1.01 | 1.01 | <0.0001 | 1.00 | 0.99 | 1.01 | 0.850 | 1.00 | 1.00 | 1.01 | 0.229 |
| Gender | ||||||||||||||||
| Male | Ref | Ref | Ref | Ref | Ref | Ref | Ref | Ref | Ref | Ref | Ref | Ref | ||||
| Female | 0.96 | 0.88 | 1.05 | 0.388 | 0.92 | 0.87 | 0.97 | 0.001 | 1.10 | 0.88 | 1.38 | 0.408 | 0.93 | 0.81 | 1.06 | 0.280 |
| Deyo/Charlson score a | ||||||||||||||||
| 0 | Ref | Ref | Ref | Ref | Ref | Ref | Ref | Ref | Ref | Ref | Ref | Ref | ||||
| 1 | 2.13 | 1.75 | 2.58 | <0.0001 | 1.64 | 1.50 | 1.80 | <0.0001 | 2.83 | 1.60 | 4.99 | 0.000 | 1.70 | 1.32 | 2.19 | <0.0001 |
| ≥2 | 3.38 | 2.76 | 4.13 | <0.0001 | 2.28 | 2.05 | 2.54 | <0.0001 | 3.83 | 2.15 | 6.82 | <0.0001 | 2.09 | 1.57 | 2.77 | <0.0001 |
| Comorbidities | ||||||||||||||||
| Obesityb | 0.70 | 0.61 | 0.80 | <0.0001 | 0.77 | 0.71 | 0.83 | <0.0001 | 0.63 | 0.44 | 0.90 | 0.010 | 0.71 | 0.58 | 0.87 | 0.001 |
| History of hypertensionb | 1.00 | 0.90 | 1.10 | 0.952 | 1.00 | 0.94 | 1.06 | 0.963 | 1.02 | 0.79 | 1.32 | 0.883 | 0.89 | 0.77 | 1.04 | 0.145 |
| History of diabetesb | 0.98 | 0.88 | 1.09 | 0.666 | 0.91 | 0.86 | 0.98 | 0.007 | 1.07 | 0.82 | 1.39 | 0.629 | 1.01 | 0.86 | 1.20 | 0.883 |
| Heart failuref | 1.35 | 1.21 | 1.51 | <0.0001 | 1.08 | 1.01 | 1.16 | 0.034 | 1.39 | 1.04 | 1.87 | 0.028 | 1.11 | 0.92 | 1.34 | 0.290 |
| History of chronic pulmonary diseaseb | 1.16 | 1.05 | 1.28 | 0.003 | 0.96 | 0.90 | 1.02 | 0.175 | 1.01 | 0.78 | 1.31 | 0.918 | 0.87 | 0.74 | 1.02 | 0.092 |
| Peripheral vascular diseaseb | 1.12 | 0.96 | 1.31 | 0.154 | 1.13 | 1.03 | 1.24 | 0.013 | 1.75 | 1.25 | 2.47 | 0.001 | 1.34 | 1.07 | 1.68 | 0.010 |
| Renal failure | 1.00 | 0.88 | 1.14 | 0.983 | 0.91 | 0.84 | 0.99 | 0.027 | 1.38 | 0.99 | 1.91 | 0.054 | 1.20 | 0.97 | 1.49 | 0.098 |
| Neurological disorder or paralysis | 0.90 | 0.78 | 1.05 | 0.174 | 1.16 | 1.07 | 1.25 | 0.000 | 0.76 | 0.50 | 1.14 | 0.177 | 1.14 | 0.92 | 1.41 | 0.219 |
| Anemiab | 1.30 | 1.19 | 1.44 | <0.0001 | 1.46 | 1.38 | 1.55 | <0.0001 | 1.14 | 0.90 | 1.45 | 0.264 | 1.04 | 0.90 | 1.21 | 0.584 |
| Hematological or oncological malignancyb | 3.29 | 2.91 | 3.73 | <0.0001 | 1.81 | 1.67 | 1.96 | <0.0001 | 3.56 | 2.56 | 4.95 | <0.0001 | 1.83 | 1.48 | 2.26 | <0.0001 |
| Rheumatoid arthritis or another collagen vascularb | 1.17 | 0.95 | 1.44 | 0.130 | 1.05 | 0.92 | 1.19 | 0.484 | 0.92 | 0.48 | 1.74 | 0.788 | 1.13 | 0.80 | 1.60 | 0.495 |
| Depression, psychosis, or substance abuseb | 1.00 | 0.89 | 1.12 | 0.972 | 1.12 | 1.05 | 1.19 | 0.001 | 0.81 | 0.59 | 1.13 | 0.211 | 1.07 | 0.89 | 1.28 | 0.470 |
| Median household income category for patient's zip code c | ||||||||||||||||
| 0‐25th percentile | Ref | Ref | Ref | Ref | Ref | Ref | Ref | Ref | Ref | Ref | Ref | Ref | ||||
| 26‐50th percentile | 0.89 | 0.79 | 1.01 | 0.066 | 0.94 | 0.88 | 1.01 | 0.098 | 0.87 | 0.64 | 1.19 | 0.382 | 0.93 | 0.77 | 1.11 | 0.416 |
| 51‐75th percentile | 0.90 | 0.80 | 1.02 | 0.096 | 0.92 | 0.86 | 0.99 | 0.027 | 0.86 | 0.62 | 1.17 | 0.333 | 0.89 | 0.74 | 1.08 | 0.236 |
| 76‐100th percentile | 1.03 | 0.91 | 1.17 | 0.601 | 0.91 | 0.84 | 0.98 | 0.015 | 0.97 | 0.71 | 1.33 | 0.859 | 0.84 | 0.69 | 1.03 | 0.093 |
| Primary payer | ||||||||||||||||
| Medicare/Medicaid | Ref | Ref | Ref | Ref | Ref | Ref | Ref | Ref | Ref | Ref | Ref | Ref | ||||
| Private including HMO | 0.94 | 0.83 | 1.06 | 0.311 | 0.84 | 0.78 | 0.90 | <0.0001 | 1.06 | 0.79 | 1.42 | 0.693 | 0.98 | 0.82 | 1.18 | 0.835 |
| Self‐pay/no charge/other | 0.77 | 0.61 | 0.98 | 0.030 | 0.91 | 0.81 | 1.03 | 0.137 | 1.04 | 0.59 | 1.86 | 0.883 | 0.92 | 0.65 | 1.31 | 0.649 |
| Hospital characteristics | ||||||||||||||||
| Hospital bed size d | ||||||||||||||||
| Small | Ref | Ref | Ref | Ref | Ref | Ref | Ref | Ref | Ref | Ref | Ref | Ref | ||||
| Medium | 0.98 | 0.85 | 1.14 | 0.795 | 1.10 | 1.01 | 1.21 | 0.036 | 0.98 | 0.65 | 1.47 | 0.918 | 1.19 | 0.92 | 1.54 | 0.193 |
| Large | 1.03 | 0.90 | 1.18 | 0.711 | 1.13 | 1.04 | 1.23 | 0.004 | 1.09 | 0.76 | 1.56 | 0.658 | 1.39 | 1.09 | 1.76 | 0.007 |
| Hospital teaching status e | ||||||||||||||||
| Non‐teaching | Ref | Ref | Ref | Ref | Ref | Ref | Ref | Ref | Ref | Ref | Ref | Ref | ||||
| Teaching | 1.17 | 1.07 | 1.28 | 0.001 | 1.18 | 1.12 | 1.24 | <0.0001 | 1.20 | 0.95 | 1.52 | 0.134 | 1.11 | 0.96 | 1.28 | 0.156 |
| Admission day | ||||||||||||||||
| Weekdays | Ref | Ref | Ref | Ref | Ref | Ref | Ref | Ref | Ref | Ref | Ref | Ref | ||||
| Weekend | 0.99 | 0.89 | 1.10 | 0.858 | 1.02 | 0.96 | 1.09 | 0.455 | 1.05 | 0.80 | 1.37 | 0.729 | 1.19 | 1.02 | 1.39 | 0.027 |
| Disposition | ||||||||||||||||
| Home | Ref | Ref | Ref | Ref | Ref | Ref | Ref | Ref | Ref | Ref | Ref | Ref | ||||
| Facility | 1.60 | 1.45 | 1.78 | <0.0001 | 1.46 | 1.37 | 1.56 | <0.0001 | 1.43 | 1.10 | 1.85 | 0.008 | 1.34 | 1.14 | 1.57 | <0.001 |
| Length of stay | Ref | Ref | Ref | |||||||||||||
| ≤2 | Ref | Ref | Ref | Ref | Ref | Ref | Ref | Ref | Ref | |||||||
| 3‐6 | 1.47 | 1.28 | 1.68 | <0.0001 | 1.41 | 1.31 | 1.53 | <0.0001 | 1.30 | 0.93 | 1.82 | 0.128 | 1.32 | 1.07 | 1.61 | 0.008 |
| >6 | 2.14 | 1.86 | 2.47 | <0.0001 | 2.43 | 2.23 | 2.64 | <0.0001 | 2.22 | 1.57 | 3.14 | <0.0001 | 2.49 | 2.02 | 3.07 | <0.0001 |
Abbreviation: HMO, Health Maintenance Organization; HR, Hazard Ratio; UL, Upper Limit; LL, Lower Limit.
Charlson/Deyo comorbidity index (CCI) was calculated as per Deyo classification.
Variables are AHRQ comorbidity measures.
Represents a quartile classification of the estimated median household income of residents in the patients ZIP code, derived from ZIP code‐demographic data obtained from Claritas. The quartiles are identified by values of 1 to 4, indicating the poorest to wealthiest populations. Because these estimates are updated annually, the value ranges vary by year. https://www.hcup-us.ahrq.gov/db/vars/zipinc_qrtl/nrdnote.jsp.
The bed size cutoff points divided into small, medium, and large have been done so that approximately one‐third of the hospitals in a given region, location, and teaching status combination would fall within each bed size category. https://www.hcup-us.ahrq.gov/db/vars/hosp_bedsize/nrdnote.jsp.
A hospital is considered to be a teaching hospital if it has an AMA‐approved residency program, is a member of the Council of Teaching Hospitals (COTH) or has a ratio of full‐time equivalent interns and residents to beds of 0.25 or higher. https://www.hcup-us.ahrq.gov/db/vars/hosp_ur_teach/nrdnote.jsp.
Comorbidity was identified by appropriate ICD 9 CM diagnosis codes in secondary diagnosis field.
Among patients who underwent a surgical intervention in the preceding 90 days, the risk factors for readmission with PE were very similar to the overall PE cohort. We identified 30 844 patients with surgical intervention, of which 8371 (27.4%) were readmitted within 90 days. Over half of the patients were over the age of 65 and 46.18% were males. The comorbidities associated with PE related readmissions in the surgical PE subgroup were similar to the general PE population (Table 1).
The results of the multivariate analysis for patients readmitted for surgical PE were also similar to the general population. Malignancy (HR 1.47, CI 1.33‐1.67 P < 0.0001), heart failure (HR 1.34, CI 1.22 1.47, P < 0.0001). LOS >6 days (HR 1.38, CI 1.26‐1.52, P < 0.0001) and higher Charlson/Deyo comorbidity score (Score 1 HR 1.33, CI 1.20‐1.49, P < 0.0001 and score ≥ 2 HR 1.59, CI 1.40‐1.82 P < 0.0001) were associated with the highest 90‐day readmission rates. The multivariate for 30‐day readmission also showed similar results (Table 3).
4. DISCUSSION
Our primary outcome was to find the 90‐day readmission rates and mortality in patients admitted with a primary diagnosis of PE. The 90‐day readmission rate in our study was 21.36%, which was much lower than the 58.6% 90‐readmission rates reported by Spyropoulos et al in 2007.4 Although unclear, one of the major reasons could be better treatment options with the introduction of the direct factor Xa inhibitors in 2011, which have been shown to be non‐inferior to vitamin K antagonists but with a lower bleeding risk.9, 10 With no need for weekly INR monitoring, patient compliance has also improved with direct factor Xa inhibitors.11 The all‐cause 90‐day mortality rate following first admissions was 4.92% (12 820 of 260 614 patients admitted with PE initially died) whereas the mortality rate in readmitted patients was significantly higher at 23.03%. The 30‐day all‐cause mortality in the EMPEROR‐trial was 5.4%, consistent with our analysis.12
Obesity was associated with a lower risk of 90‐day readmission (HR 0.86, CI 0.83‐0.89, P < 0.001) and mortality (HR 0.70, CI 0.61‐0.80, P < 0.001). Although, several studies have shown obesity to be an independent predictor for pulmonary embolism, the mortality rate remains low.13, 14 This “obesity paradox” has been seen in several other diseases, especially heart failure.15, 16 The mechanism behind this is not clearly understood.
Malignancy increased the risk of 90‐day readmission (HR 1.63, CI 1.56‐1.70, P < 0.001). About 8.31% of the 90‐day readmissions were related to malignancy with solid tumors contributing 7.49% of these readmissions. Malignancy itself is an independent risk factor for VTE with an odds ratio as high as 6%.17 While the HR of 90‐day mortality and bleeding was 1.81 in our study, older studies have showed a bleeding HR ranging from 0.9% to 4.5%.18
A study by Heit et al also showed limb paralysis to be an independent risk factor for VTE (OR 3, 95% CI 1.3‐7.4).17 We found that neurological disorders were associated with a higher risk of readmission, constituting 4% of the readmissions. Although likely, no relationship was established between immobilization and the increased risk of readmission for PE because of the small number of patients in this cohort.
A history of chronic pulmonary disease was also associated with a higher risk of 90‐day readmission and mortality. They had a 16% higher risk of readmission compared to patients without pulmonary disease. A Canadian meta‐analysis showed that up to 1 in 4 patients hospitalized for COPD could have a PE.19 This was confirmed by another French study, which showed a 25% prevalence of PE in patients admitted with severe COPD.20 Several other studies have shown COPD to be an independent risk factor for PE.21 With such high prevalence of PE in COPD patients, clinicians should have a low threshold to screen for PE in the appropriate clinical setting. COPD exacerbations contributed to 2.60% of the 90‐day readmissions. Respiratory infections have also been associated with an increased risk of PE.22 Respiratory infections accounted for 2.43% of all 90‐day readmissions in our analysis. Urinary tract infections have also been associated with a higher risk of PE. The risk reduced to baseline after 1 year.22
Ninety‐day readmissions due to recurrence of PE were 7.33%. There are no recent data regarding PE recurrence rates after the use of NOACs. The 2‐year recurrence rate of venous thromboembolism (DVT and PE) was 7.7% per year.23 The 7, 30, and 180‐day recurrence rates after an initial episode of VTE were 1.6%, 5.2%, and 10.1%.17 The ICOPER study in 1999 showed a 7.9% recurrence rate of PE at 3 months. Our results were consistent with these findings (7.33% recurrence rate at 90‐days).21
Bleeding is an important complication of anticoagulation treatment. We classified major bleeding as any intracranial bleed or gastrointestinal bleeding. All other bleeding complications were classified as “other bleeds.” The incidence of intracranial bleeding in patients readmitted with PE was 0.25% and gastrointestinal bleeds was 2.18%. The incidence of patients readmitted with any bleed was 2.99%. Intracranial bleeds contributed 0.82% of readmissions; GI bleeding 3.68% and any bleeding contributed 2.25% of total 90‐day readmissions. The 1996 ISCOAT trial showed an all‐cause bleeding incidence due to of 6.8 per 3100 patient years. The study population was on warfarin or acenocoumarol.24 With the introduction of direct factor Xa inhibitors, the incidence of major bleeding has decreased significantly compared with conventional vitamin K antagonist agents.9, 10 The oral direct thrombin inhibitor used in PE treatment also has a reversal agent now, thus improving the safety of these medications.25 The 90‐day mortality and major bleeding rates were significantly higher in patients with a higher Charlson/Deyo comorbidity score ≥ 2, anemia, hemato‐oncologic malignancy, and patients discharged to a nursing facility. Obesity was associated with lower 90‐day mortality and major bleeding rates.
The number of patients who died from PE in 2013 was 12 820, which translated into a mortality rate of 4.93%. In 1979, the number of people who died from PE was estimated at 35 754 falling to 24 947 in 1998.26 The development of newer diagnostic techniques, newer medications, lower bleeding rates, and risk stratification scores for PE treatment may be associated with this decrease in mortality from 1979 to 2013.27 Our findings were also consistent with Kohn et al who showed similar 30‐ and 90‐day mortality rates and 90‐day readmissions using the Veterans Health Administration database.28
There are several limitations to this study. It is based on the National Readmission Database from the HCUP; although HCUP is well‐designed, the data is limited by coding errors in the primary and readmission coding. Ninety‐day readmission rates could not be calculated for patients hospitalized in the last 3 months of the calendar year, leaving approximately one quarter of admissions. Despite this, this is the largest population study for pulmonary embolism in the recent years incorporating data from 22 states accounting for 51.2% of total US population and 49.3% of all US hospitalizations.29 Considering DRG code suggest either procedural status of admission or main diagnosis of admission, it would not be possible to identify primary cause of each readmission using DRG codes. We decided to use ICD‐9 codes in primary diagnosis field to identify causes of readmission. Similar methodology was used by multiple researchers in multiple journals in past.
In conclusion, PE is a common cause of morbidity and mortality in hospitalized patients. With the recent advances in diagnosis and treatment, the morbidity and mortality has decreased significantly. The 90‐day mortality and bleeding rates have decreased significantly in the recent years with use of newer anticoagulants and risk stratification scores. Higher Charlson comorbidity index scores and women had a higher risk of readmission. Chronic pulmonary disease, malignancy, anemia, and longer length of stay during the primary admission also increased the risk of readmission. Malignancies, pulmonary diseases especially COPD, infections and bleeding disorders were the most common etiologies of readmission.
Supporting information
FIGURE S1. Flowchart of study population
FIGURE S2.
FIGURE S3.
FIGURE S4.
TABLE S1. Coding and ICD 9 codes of causes of readmission
TABLE S2. Deyo's modification of Charlson's comorbidity index (CCI)
TABLE S3. Post hoc analysis of 30‐day readmission
ACKNOWLEDGMENT
No study specific funding was used to support this work. The authors are solely responsible for the study design, conduct and analyses, drafting and editing of the manuscript and its final contents. All authors had access to the data and a role in writing the manuscript.
Conflict of interest
The authors declare no potential conflict of interests.
Shah P, Arora S, Kumar V, et al. Short‐term outcomes of pulmonary embolism: A National Perspective. Clin Cardiol. 2018;41:1214–1224. 10.1002/clc.23048
REFERENCES
- 1. Silverstein MD, Heit JA, Mohr DN, Petterson TM, O'Fallon WM, Melton LJ. Trends in the incidence of deep vein thrombosis and pulmonary embolism: a 25‐year population‐based study. Arch Intern Med. 1998;158(6):585‐593. [DOI] [PubMed] [Google Scholar]
- 2. Wiener RS, Schwartz LM, Woloshin S. Time trends in pulmonary embolism in the United States evidence of overdiagnosis. Arch Intern Med. 2011;171(9):831‐837. 10.1001/archinternmed.2011.178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Hawkins D. Pharmacoeconomics of thrombosis management. Pharmacotherapy. 2004;24:95S‐99S. [DOI] [PubMed] [Google Scholar]
- 4. Spyropoulos A, Lin J. Direct medical costs of venous thromboembolism and subsequent hospital readmission rates: an administrative claims analysis from 30 managed care organizations. J Manag Care Pharm. 2007;13:475‐486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kearon C, Akl EA, Comerota AJ, et al. Antithrombotic therapy for VTE disease: antithrombotic therapy and prevention of thrombosis, 9th ed: American college of chest physicians evidence‐based clinical practice guidelines. Chest. 2012;141:e419S‐e530S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Agency for Healthcare Research and Quality. HCUP Nationwide Readmissions Database. HCUP. https://www.hcup-us.ahrq.gov/nrdoverview.jsp. Accessed August 8.
- 7. HCaUP . Comorbidity Software.Version 3.7. 2008. 2016.
- 8. Deyo RA, Cherkin DC, Ciol MA, et al. Adapting a clinical comorbidity index for use with ICD‐9‐CM administrative databases. J Clin Epidemiol. 1992;45:613‐619. [DOI] [PubMed] [Google Scholar]
- 9. Investigators E‐P , Buller HR, Prins MH, et al. Oral rivaroxaban for the treatment of symptomatic pulmonary embolism. N Engl J Med. 2012;366:1287‐1297. 10.1056/NEJMoa1113572. [DOI] [PubMed] [Google Scholar]
- 10. Agnelli G, Buller HR, Cohen A, et al. Oral apixaban for the treatment of acute venous thromboembolism. N Engl J Med. 2013;369:799‐808. 10.1056/NEJMoa1302507. [DOI] [PubMed] [Google Scholar]
- 11. Prins MH, Bamber L, Cano SJ, et al. Patient‐reported treatment satisfaction with oral rivaroxaban versus standard therapy in the treatment of pulmonary embolism; results from the EINSTEIN PE trial. Thromb Res. 2015;135:281‐288. [DOI] [PubMed] [Google Scholar]
- 12. Pollack CV, Schreiber D, Goldhaber SZ, et al. Clinical characteristics, management, and outcomes of patients diagnosed with acute pulmonary embolism in the emergency department: initial report of EMPEROR (multicenter emergency medicine pulmonary embolism in the real world registry). J Am Coll Cardiol. 2011;57(6):700‐706. [DOI] [PubMed] [Google Scholar]
- 13. Stein PD, Beemath A, Olson RE. Obesity as a risk factor in venous thromboembolism. Am J Med. 2005;118(9):978‐980. [DOI] [PubMed] [Google Scholar]
- 14. Goldhaber SZ, Savage DD, Garrison RJ, et al. Risk factors for pulmonary embolism: the Framingham study. Am J Med. 1983;74(6):1023‐1028. [DOI] [PubMed] [Google Scholar]
- 15. Fleischmann E, Teal N, Dudley J, et al. Influence of excess weight on mortality and hospital stay in 1346 hemodialysis patients. Kidney Int. 1999;55:1560‐1567. [DOI] [PubMed] [Google Scholar]
- 16. Curtis JP, Selter JG, Wang Y, et al. The obesity paradox: body mass index and outcomes in patients with heart failure. Arch Intern Med. 2005;165(1):55‐61. [DOI] [PubMed] [Google Scholar]
- 17. Heit JA, Mohr DN, Silverstein MD, Petterson TM, O'Fallon WM, Melton LJ. Predictors of recurrence after deep vein thrombosis and pulmonary embolism: a population‐based cohort study. Arch Intern Med. 2000;160(6):761‐768. [DOI] [PubMed] [Google Scholar]
- 18. Prandoni P, Lensing AW, Piccioli A, et al. Recurrent venous thromboembolism and bleeding complications during anticoagulant treatment in patients with cancer and venous thrombosis. Blood. 2002;100:3484‐3488. [DOI] [PubMed] [Google Scholar]
- 19. Rizkallah J, Man SFP, Sin DD. Prevalence of pulmonary embolism in acute exacerbations of COPD: a systematic review and metaanalysis. Chest. 2009;135(3):786‐793. [DOI] [PubMed] [Google Scholar]
- 20. Tillie‐Leblond I, Marquette CH, Perez T, et al. Pulmonary embolism in patients with unexplained exacerbation of chronic obstructive pulmonary disease: prevalence and risk factors. Ann Intern Med. 2006;144(6):390‐396. [DOI] [PubMed] [Google Scholar]
- 21. Goldhaber SZ, Visani L, De Rosa M, et al. Acute pulmonary embolism: clinical outcomes in the international cooperative pulmonary embolism registry (ICOPER). Lancet. 1999;353(9162):1386‐1389. [DOI] [PubMed] [Google Scholar]
- 22. Smeeth L, Cook C, Thomas S, Hall AJ, Hubbard R, Vallance P. Risk of deep vein thrombosis and pulmonary embolism after acute infection in a community setting. Lancet. 2006;367(9516):1075‐1079. [DOI] [PubMed] [Google Scholar]
- 23. Cushman M, Tsai AW, White RH, et al. Deep vein thrombosis and pulmonary embolism in two cohorts: the longitudinal investigation of thromboembolism etiology. Am J Med. 2004;117(1):19‐25. [DOI] [PubMed] [Google Scholar]
- 24. Palareti G, Leali N, Coccheri S, et al. Bleeding complications of oral anticoagulant treatment: an inception‐cohort, prospective collaborative study (ISCOAT). Lancet. 1996;348(9025):423‐428. [DOI] [PubMed] [Google Scholar]
- 25. Glund S, Stangier J, Schmohl M, et al. Idarucizumab, a specific antidote for dabigatran: immediate, complete and sustained reversal of dabigatran induced anticoagulation in elderly and renally impaired subjects. Blood. 2014;124(21):344‐344.24914142 [Google Scholar]
- 26. Horlander KT, Mannino DM, Leeper KV. Pulmonary embolism mortality in the United States, 1979‐1998: an analysis using multiple‐cause mortality data. Arch Intern Med. 2003;163(14):1711‐1717. [DOI] [PubMed] [Google Scholar]
- 27. Tapson VF. Advances in the diagnosis and treatment of acute pulmonary embolism. F1000 Med Rep. 2012;4:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kohn CG, Weeda ER, Kumar N, et al. External validation of a claims‐based and clinical approach for predicting post‐pulmonary embolism outcomes among United States veterans. Intern Emerg Med. 2017;12(5):613‐619. [DOI] [PubMed] [Google Scholar]
- 29. https://www.hcup-us.ahrq.gov/nrdoverview.jsp
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
FIGURE S1. Flowchart of study population
FIGURE S2.
FIGURE S3.
FIGURE S4.
TABLE S1. Coding and ICD 9 codes of causes of readmission
TABLE S2. Deyo's modification of Charlson's comorbidity index (CCI)
TABLE S3. Post hoc analysis of 30‐day readmission


