Abstract
Isolated cardiac sarcoidosis is a generally accepted disease condition, and the low yield of endomyocardial biopsy because of patchy involvement is also well known. However, current guidelines still require histologic confirmation of granuloma for the diagnosis of cardiac sarcoidosis, either in myocardial or extra‐cardiac tissues. Therefore, only a presumptive diagnosis of chronic multifocal myocarditis of unknown origin can be made in a large number of patients in whom the only considerable diagnosis is cardiac sarcoidosis based on current knowledge. Even if these patients are treated with the same treatment scheme as that for cardiac sarcoidosis, which may not cause harm in the absence of a definite diagnosis, the true spectrum of cardiac sarcoidosis could not be determined for deciding the optimal treatment strategy. In addition, the current recommendations for dose, duration of initial steroid therapy, and treatment in patients who did not respond to initial steroid therapy are not easy to follow in real‐world practice. We would like to propose a scoring system for the diagnosis of cardiac sarcoidosis and suggest our adoption or modification of the diverse current recommendations.
Keywords: cardiac sarcoidosis, histologic diagnosis, imaging tests, steroid therapy
1. INTRODUCTION
Sarcoidosis is a multisystem granulomatous disease characterized by the presence of noncaseating granuloma in the involved organ.1, 2 Except for a rare acute presentation,3, 4 sarcoidosis is a chronic progressive disease, most frequently involving the lungs. Despite the higher incidence of myocardial involvement in autopsy series,5 clinically apparent cardiac sarcoidosis is reported to be seen in only 5% to 10% of patients with systemic sarcoidosis.6, 7
Cardiac involvement can be diagnosed directly with endomyocardial biopsy. However, myocardial involvement in sarcoidosis is patchy in nature rather than diffuse. Therefore, the diagnostic yield of endomyocardial biopsy has been reported to be only approximately 20%.8, 9 Thus, in most cases, the diagnosis of cardiac sarcoidosis is made when the patient demonstrates histologically confirmed extracardiac sarcoidosis and has nonhistologic clues of cardiac involvement. These patients are called the “clinical diagnosis group” in widely cited articles.10, 11, 12 The strategy works only in the presence of extracardiac sarcoidosis; however, several studies have suggested the presence of isolated cardiac sarcoidosis7, 13, 14 in which the diagnosis of extracardiac sarcoidosis cannot be made. Therefore, diagnostic criteria for isolated cardiac sarcoidosis not requiring histologic confirmation of noncaseating granuloma in the myocardium are needed.
In addition to the clinical dilemma in diagnosing cardiac sarcoidosis, various therapeutic issues should be raised for discussion, including the (a) usefulness of steroid treatment in cardiac sarcoidosis, (b) initial dose of steroid and treatment strategy, (c) effect of steroid on left ventricular (LV) function and atrioventricular (AV) conduction recovery, and (d) indication for implantable cardioverter defibrillator (ICD) insertion.
2. DIAGNOSIS
2.1. Clinical need for new criteria for cardiac sarcoidosis
Nowadays, isolated cardiac sarcoidosis is a generally accepted disease condition. Kandolin et al13 reported that 63% of cardiac sarcoidosis cases showed isolated myocardial involvement. They only included histologically confirmed cases, including some explanted cases during transplantation or autopsy. Had they included patients with cardiac sarcoidosis in whom histologic confirmation could not be made, the proportion of isolated cardiac sarcoidosis might have increased further.
We prospectively collected 27 patients whose clinical and laboratory findings suggest chronic multifocal patchy myocarditis without other possible causes except cardiac sarcoidosis. A total of 24 patients underwent biopsy. Notably, a single patient may undergo one or two biopsy procedures. Of these patients, 1 underwent liver biopsy, 11 underwent hilar lymph node (LN) biopsy, and 21 underwent endomyocardial biopsy. Among these patients, granulomas were found in the liver biopsy of a patient who was suspected to have liver involvement, whereas one patient showed granuloma and another patient showed scattered histiocytes in 11 hilar LN biopsies. Of the 21 patients who underwent endomyocardial biopsy, granuloma was observed only in one patient (1 of 21, 4%).
Hilar LN is the most likely site for the histological diagnosis of extracardiac sarcoidosis. Twenty‐five patients were screened for the presence of hilar LN enlargement; however, only 10 patients (40%) showed hilar LN enlargement (the presence of any LN ≥ 1 cm in diameter). Granuloma was seen only in one patient, and another patient showed scattered histocytes.
If the diagnosis is made solely on the basis of most recent guidelines from the Heart Rhythm Society (HRS),12 only three patients can be diagnosed as having cardiac sarcoidosis (assuming scattered histiocytes in hilar LN biopsy equivalent to the presence of granuloma in the diagnosis of sarcoidosis); hence, 24 patients were classified as having unknown inflammatory myocarditis. The guidelines suggested by the Japanese Ministry of Health and Welfare (JMH)10 and later revised by the Japanese Society of Sarcoidosis and Other Granulomatous Disorders (JSSOG),11 allow clinical diagnosis and histologic confirmation for the presence of extracardiac sarcoidosis. However, the known clinical symptoms and signs that suggest a diagnosis of extracardiac sarcoidosis are rather scarce and insufficient. Two patients complained of Raynaud's phenomenon, and another patient was suspected to have interstitial lung disease based on chest CT findings; however, these conditions were insufficient to make a diagnosis of extracardiac sarcoidosis.
The criteria suggested by the JMH/JSSOG and HRS did not consider the presence of isolated cardiac sarcoidosis. The recently suggested electrogram‐guided biopsy may increase the yield of endomyocardial biopsy, which may solve the current problem in the diagnosis of isolated cardiac sarcoidosis. However, at present, it seems useful to identify relatively diffuse lesions as fibrosis in myocarditis or fatty infiltration in arrhythmogenic right ventricular dysplasia (ARVD), rather than specifically identifying a granuloma.15 Therefore, clinically relevant criteria especially in diagnosing isolated cardiac sarcoidosis are needed. In our opinion, for patients suspected of having chronic inflammatory myocarditis and who showed patchy involvement at multiple locations, without other suspected possible causes, diagnosis of probable cardiac sarcoidosis can be reasonably made. During the period when we prospectively collected 27 patients, 7 patients underwent the same diagnostic workup. In these seven patients, we could not confidently diagnose multifocal patch inflammation mainly because some test results were equivocal. We scored positive finding on an individual imaging study, focal fibrosis in endomyocardial biopsy, and findings suggesting systemic involvement as 1, assuming the equivocal finding as positive. Among the imaging studies, we weighed 18‐Fluoro‐2‐deoxyglucose positron emission tomography (FDG‐PET) findings, which were scored either 1 or 2. Among these 27 patients, score was 4 or higher, and less than 4 in 7 patients. Score of 4 or higher in diagnosing cardiac sarcoidosis might be higher than optimal, which may lower the sensitivity and let the patients undergo unnecessary diagnostic tests. However, in preceeding to treatment, we think that specificity is more important than low sensitivity or performing unnecessary tests (Figure 1).
Figure 1.
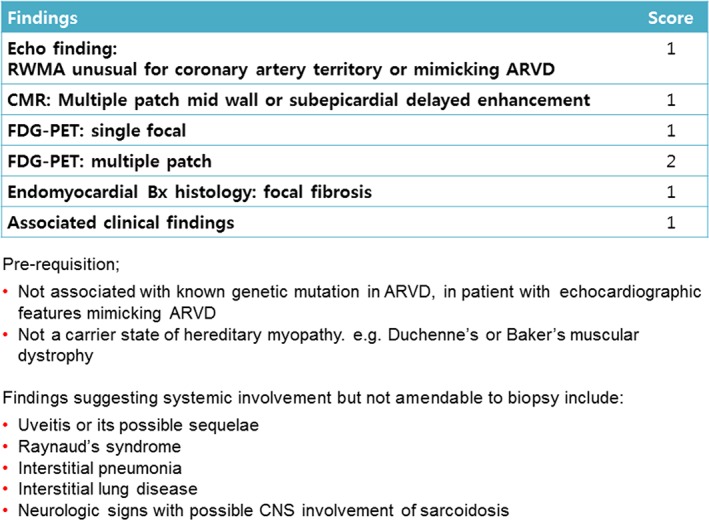
Criteria for probable cardiac sarcoidosis. ARVD, arrhythmogenic right ventricular dysplasia; CMR, cardiac magnetic resonance; CNS, central nervous system; FDG‐PET, 18‐Fluoro‐2‐deoxyglucose positron emission tomography; RWMA, regional wall motion abnormality
2.2. Electrocardiogram(EKG)
In addition to the several nonspecific EKG findings, patients with cardiac sarcoidosis may experience symptomatic conduction disturbances or ventricular arrhythmias. Holter monitoring is sometimes needed to reveal the electrocardiographic abnormality that is not apparent with resting 12‐lead EKG. In the diagnostic viewpoint, EKG findings have been widely neglected as they have poor sensitivity. The presence of epsilon waves does not necessarily lead to the diagnosis of arrhythmogenic right ventricular dysplasia(ARVD), which is reportedly seen in patients with cardiac sarcoidosis.16 However, minute EKG findings can sometimes lead to further diagnostic tests and can result in a diagnosis of cardiac sarcoidosis; otherwise, the disease might be missed (Figure 2).
Figure 2.
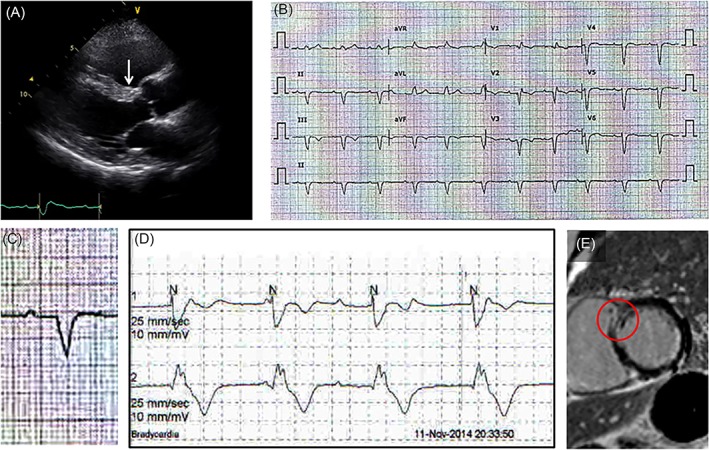
Clinical usefulness of resting EKG finding. A, Wall motion abnormality at the basal anterior septum (arrow) was not apparent. Only high echogenicity compared with the rest of the anterior septum is noted. B and C, Resting EKG showing first‐degree AV block with a wide QRS complex. As a cause of PR prolongation, the possibility of a tri‐fascicular block should be considered. D, Holter monitor showing intermitted complete AV block with wide QRS escape beats suggesting an infra‐His block. Therefore, it is not relevant to think that a first‐degree AV block as a conduction delay at the AV node and an infra‐His AV block exist separately. E, EKG findings in this patient suggest that a certain pathologic lesion is most likely present at the basal anterior septum (arrow in A). Therefore, even the tiny mid‐wall delayed enhancement at the basal anterior septum shown here, otherwise neglected, should be considered as a pathologic delayed enhancement. AV, atriventricular; EKG, electrocardiogram; PR, PR interval; QRS, QRS complex
2.3. Echocardiogram
Except for very rare cases of increased LV wall thickness mimicking hypertrophic cardiomyopathy,17, 18 the classic echocardiographic finding is multiple regional wall motion abnormalities (RWMAs) that do not match the coronary artery territory, which indicates nonischemic multiple RWMAs. Depending on the extent of sarcoid involvement, multiple patchy sarcoid involvement does not always result in multiple RWMAs. Moreover, the nonischemic nature of RWMAs based on the unusual coronary artery territory is rather difficult to determine even for an expert echocardiographer, especially when the RWMA is confined to a single coronary artery territory. CT coronary angiography and cardiac magnetic resonance (CMR) imaging are helpful in this situation. CMR provides direct evidence of multiple patchy nonischemic involvement of the myocardium, in contrast to the indirect evidence of the nonischemic nature of RWMA based on a normal CT coronary angiogram. However, a CT coronary angiogram can provide additional information about the presence of hilar lymphadenopathy and the possible site for biopsy.
Among the RWMA, thinning of the basal anterior septum seems to be rather specific to cardiac sarcoidosis, although the exact specificity of this finding has not been elucidated. However, considering only at this echocardiographic finding may lead to missed diagnosis in a large number of patients, as the reported incidence of this finding is only 20% to 28%.19, 20 In our 27 patients, the involvement of the basal anterior septum, including scarring and thinning (Figure 3), had a much higher incidence of 48% (13 of 27).
Figure 3.
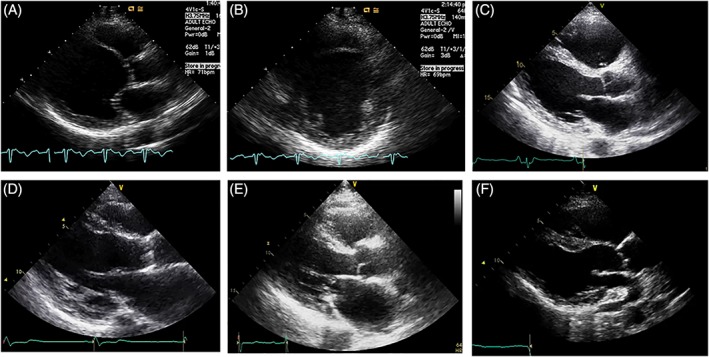
Involvement of the basal anterior septum in cardiac sarcoidosis. A and B, Thinning of the anterior septum at the mid‐ventricular and basal levels. C, Wall thinning is not apparent. Only high echogenicity is noted. D and E, In addition to wall motion, subendocardial or transmural fibrosis is suspected. F, Wall thinning limited to the basal anterior septum
In addition to the role of echocardiography in the diagnosis of cardiac sarcoidosis, echocardiography is mandatory for monitoring LV function in patients with cardiac sarcoidosis.
Recently, several studies21, 22 have reported diagnostic ambiguity between cardiac sarcoidosis and ARVD. Therefore, in cases echocardiographically suggestive of ARVD, the possibility of cardiac sarcoidosis should be considered, and histologic or genetic evidence should be sought for the differential diagnosis. In one patient with cardiac sarcoidosis who underwent cardiac transplantation, serial echocardiography before transplantation showed that the right ventricular (RV) morphology became similar to that of ARVD over time, and histological examination of the explanted heart showed fatty tissue replacement mimicking ARVD (Figure 4).
Figure 4.
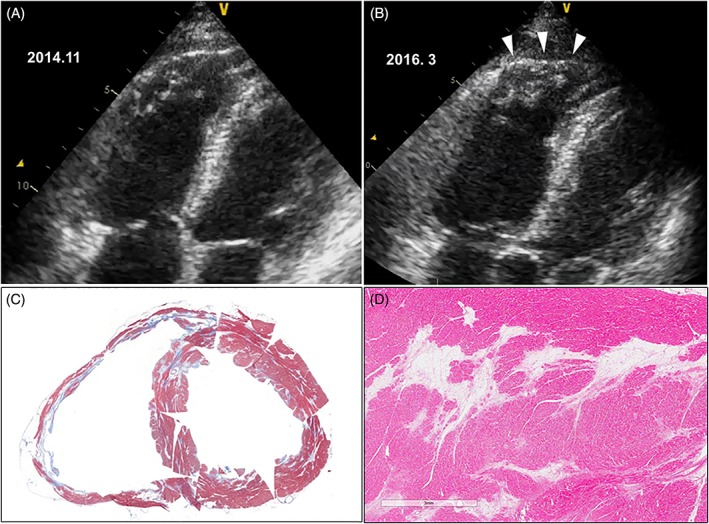
A and B, Difference in echocardiographic features 16 months apart. In addition to the slightly enlarged RV, the RV apex showed aneurysmal bulging during systole (arrowheads) mimicking ARVD. C, Explanted heart after cardiac transplantation. The myocardium stained blue (Masson trichrome stain) suggesting focal fibrosis. In addition, large areas of fatty tissue replacement mimicking ARVD are seen in the RV and left ventricle. D, High‐power view of fatty tissue replacement. ARVD, arrhythmogenic right ventricular dysplasia; RV, right ventricle
2.4. Cardiac magnetic resonance
In addition to the evaluation of wall motion in cine CMR, multiple nonischemic pattern delayed enhancements (DEs)23, 24 are useful in the diagnosis of cardiac sarcoidosis. It is not unusual to see DEs in segments without RWMA. Therefore, DE evaluation seems more sensitive than other methods used in the past to detect cardiac involvement25 in sarcoidosis.
Applications of various techniques in CMR26 have been studied in cardiac sarcoidosis, such as the presence of myocardial edema in T2‐weighted images or T1 mapping to further characterize and quantitate myocardial fibrosis.27 However, despite the large number of publications suggesting the usefulness of these techniques, whether these techniques significantly upgrade the usefulness of CMR in real‐world practice remains to be seen.
In addition to its diagnostic utility, the usefulness of CMR in the assessment of arrhythmic risk and evaluation of response to therapy is a hot topic of recent investigations.
2.5. 18‐Fluoro‐2‐deoxyglucose positron emission tomography
Unusual utilization of glucose as an energy source by the myocardium after prolonged fasting or a fatty diet can be seen during ischemic, inflammatory, or neoplastic conditions. Therefore, the presence of multiple patchy hot‐uptake in FDG‐PET implies multifocal myocarditis. If this finding does not indicate multiple areas of ischemia or multiple metastatic tumors, then sarcoidosis is the most likely condition that can be considered.
In the past, gallium scanning had been used for this purpose; however, nowadays, it is has almost been completely replaced by FDG‐PET. The preference for FDG‐PET over gallium scan is based on sensitivity and image quality. In gallium scan, as the body handles gallium similar to ferric iron, gallium is bound in the area of inflammation or rapid cell division, not in the area of ischemia.28 However, in FDG‐PET, positivity represents unsuppressed or unusual utilization of glucose as an energy source. Therefore, use of glucose by the normal myocardium as an energy source should be adequately suppressed by prolonged fasting or a fatty diet, sometimes with heparin; however, this condition may not be adequately accomplished. Moreover, usage of glucose as an energy source can be seen in ischemia or even in other unknown conditions at the cellular level not associated with epicardial coronary artery disease, such as in hypertrophic cardiomyopathy.
Taking into consideration, these limitations and paying careful attention in obtaining images, FDG‐PET, can provide both morphologic information about patchy involvement and lesion characteristic information related to inflammation. Therefore, we rely more on FDG‐PET results than on the results of other imaging modalities.
FDG‐PET can also be used to evaluate the response to steroids (Figure 5). However, in the evaluation of the responsiveness to steroids, caution should be taken concerning the effect of steroid on false‐positive FDG‐PET results, which is probably associated with the effect of steroid on glucose metabolism.
Figure 5.
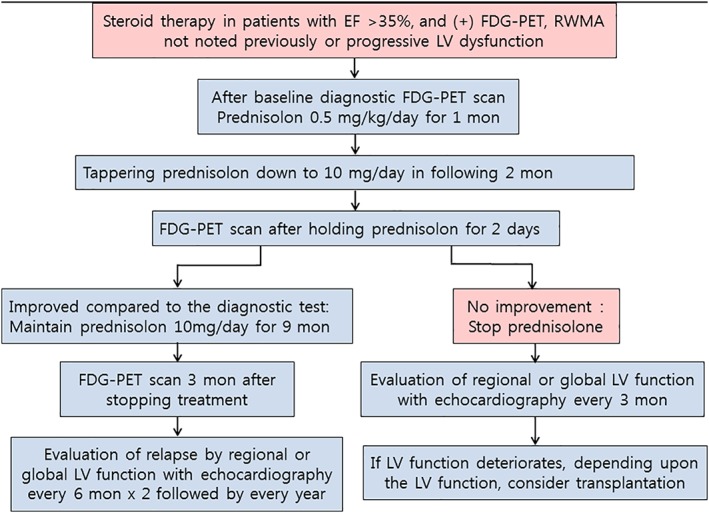
Treatment and follow‐up scheme. EF, ejection fraction; FDG‐PET, 18‐Fluoro‐2‐deoxyglucose positron emission tomography; LV, left ventricular; RWMA, regional wall motion abnormality
2.6. Serum markers
Serum angiotensin‐converting enzyme (ACE) activity is reported to be elevated in approximately 60% of patients with systemic sarcoidosis,1 which may not be the same as that noted in patients with cardiac sarcoidosis.29, 30 In one study,31 the mean value of ACE activity in 47 patients with definite cardiac sarcoidosis (16.6 ± 9.0 IU/L) fell within the normal range (≤21.4 IU/L).
Despite the disease nature of myocardial necrosis induced by inflammation, the diagnostic utility of conventional measures of evaluating myocardial damage and inflammation—troponin32, 33, 34 and high‐sensitivity C‐reactive protein (hsCRP) levels, respectively—has not gained much attention. In our 27 patients, troponin I and hsCRP levels were measured before treatment (in 14 and 16 patients, respectively). Troponin I and hsCRP levels were mildly elevated in 7 of 14 patients (50%, range 0.18‐1.45 ng/mL) and in 6 of 16 patients (38%, range 1.59‐4.59 mg/L), respectively (normal values: hsCRP 0‐0.5 mg/L, troponin I 0‐0.028 ng/mL). Additional studies are needed on the usefulness of these serum markers in the evaluation of disease activities, and therefore in the follow‐up of the disease course and treatment response.
3. TREATMENT
3.1. Usefulness of steroid treatment in cardiac sarcoidosis
The usefulness of steroids is based on the expectation of their immunologic effects on either active inflammation or the end‐result of fibrosis. Among the diseases treated with steroids, either active inflammation or fibrosis dominates. Therefore, use of steroids is not theoretically conflicting. However, granuloma represents an inflammation confined at the center, with fibroblast and collagen encasing and restricting the inflammation, thereby protecting the surrounding tissue.1 Apart from the on‐going active inflammation and the expected favorable effect of steroids during the fibrotic phase, if granuloma formation alone is considered, it cannot be confidently insisted that the fibrosis‐preventing effect of steroids is beneficial.
Yazaki et al35 showed better long‐term survival in patients using steroids. However, their study was done retrospectively in only 95 patients. Furthermore, patients whose cardiac sarcoidosis was diagnosed at autopsy were selected as the nonsteroid user group. In another study, Nagai et al36 showed a reduced composite end‐point of all‐cause death, symptomatic arrhythmias, and heart failure requiring admission with steroid therapy. However, no significant differences were found in terms of cardiac death or symptomatic arrhythmias between steroid user and nonusers, which seems to indicate that the main effect of steroid therapy is preventing the deterioration of LV function, with the effect of reducing cardiac death or symptomatic arrhythmia being small.
At our institution, we start steroid therapy once the diagnosis of cardiac sarcoidosis is made. However, we limit steroid therapy to patients who showed hot‐uptake in FDG‐PET, those who did not show RWMAs in a previous study, or those who showed a progressive nature of the disease process, to be sure that we are treating the patients in active stage. In addition, we also limit steroid therapy to patients whose LV function is relatively preserved (EF > 35%), as we do not think that steroid therapy will help in restoring LV function.
3.2. Initial dose of steroid and treatment strategy
Two different trends in the dose of initial steroid therapy exist. High‐dose of prednisolone (1 mg·kg−1·day−1) has been advocated as an initial dose37, 38 for minimal treatment failure with steroid therapy. However, in a previous study,35 the overall survival of patients treated with a high initial dose (>40 mg daily) did not differ from that in those treated with a low dose (<30 mg daily). This study was not a dose‐finding study, and dose comparison was done in only 75 patients. Moreover, the survival curves between the high‐ and low‐dose showed differences in the early phases (2‐4 years), with similar survivals after 5 years. However, for practicality, we abandoned the initial high‐dose policy because the majority of patients could not tolerate a high‐dose of steroids, and the dose should be reduced to a low dose range. In the majority of patients, remission can be induced with low‐dose steroids.
The currently suggested scheme of steroid treatment in cardiac sarcoidosis,39 except for the evaluation of response, largely adapts the scheme used in the treatment of systemic sarcoidosis.1, 40 The initial dose of therapy is maintained for 2‐3 months, which is followed by the evaluation of therapeutic response with FDG‐PET. Once there is a therapeutic response, the initial dose of steroid therapy is tapered down to maintenance dose in following 2 months and the final dose is maintained for 9 to 12 months. In our institution, before we began to collect the patients prospectively, we experienced some patients who could not tolerate 2 months of initial steroid therapy, and reduced the duration to 1 month. In those patients, we used FDG‐PET to evaluate the response to steroid therapy. The patients showed adequately suppressed disease activity, which was sustained with the maintenance dose of steroid. Since then, we have shortened the initial steroid therapy to 1 month and tapered to the maintenance dose during the following months. We have been following a scheme of 12 months as a total duration of treatment (Figure 5).
However, in the treatment strategy for patients who were confirmed to be nonresponsive to steroids based on the FDG‐PET scan after the initial steroid therapy, rather than initiating another high‐dose immunosuppressive therapy or adding a second‐line drug, we prefer not to use steroids, assuming that these patients are nonresponders. It had been our impression before this prospective collection of patients that, there were some whose clinical course remained stable for many years without steroid therapy. Furthermore, there were patients whose FDG‐PET activity spontaneously became negative. Among our 27 patients, 2 fell into this category of nonresponder and the steroid dose was rapidly tapered off as patients refused further immunosuppressive therapy. LV function was monitored thereafter, which remained stable for 1 year and 2.5 years, respectively, up to present. Even in patients who do not refuse restarting steroid therapy, we do not think that this policy is unethical. Based on the data of Nagai et al,36 the main expected difference of using steroids is the reduction in admission because of heart failure. Steroid therapy does not seem to reduce cardiac death or symptomatic arrhythmia. Therefore, we treat these patients with conventional heart failure treatment as long as the LV function is maintained.
If the LV function deteriorates, continuing high‐dose steroid therapy or adding second‐line drugs may be an option. However, even if the patient is responsive to the re‐initiation of steroid therapy, the expectation for LV function recovery is not high. Moreover, even in patients who might show improvement in LV function, the magnitude of recovery might not be sufficient41 to obviate transplantation in the near future.
3.3. Effect of steroid on LV function and AV conduction recovery
With regard to the recovery of LV function with steroid therapy, arriving at a conclusion based on current data41 is rather difficult. However, LV function would likely to be deteriorated without treatment.
Apart from steroid therapy, depending on the LV function and symptomatic status, every conventional up‐to‐date treatment for heart failure should be applied in these patients.
The more challenging issue is the treatment of conduction disturbance. In contrast to the effect of steroids on LV function, a significant proportion of patients with conduction disturbance recovers their AV conduction with steroid therapy,12, 41 which may render the inserted pacemaker useless. Despite the potential reversibility of heart block, the HRS recommended device implantation under the same indication as that for nonsarcoid patients because reversibility is unpredictable.
3.4. Ventricular tachycardia and sudden death
It is not infrequent to note nonsustained or sustained ventricular tachycardia (VT) in patients with cardiac sarcoidosis. Studies have shown divergent results42, 43, 44 on whether steroid therapy exerts a beneficial effect on VT. These results are rather expected from the theoretical viewpoint that only the right combinations between active inflammation and scar formation can result in the right milieu for the trigger and re‐entry of VT, and steroids may not only potentially disrupt but may also induce the formation of these milieu.
Ablation therapy can be an option in patient's refractory to medical therapy. In the initial reports, despite a successive ablation, the recurrence rates remained high.45, 46 Even in a recent report suggesting favorable outcome,47 recurrence rate was not dramatically reduced. Eighteen among 31 patients showed recurrence after ablation therapy, in addition to two patients who underwent a second procedure within 3 days. In this study, arrhythmia‐free survival is associated with the CMR and PET findings. Therefore, CMR and PET findings could help in selcecting more suitable patients for ablation therapy.
One of the most challenging issues in cardiac sarcoidosis is the risk of sudden death and indication for ICD insertion. Although the relationship between sudden death and cardiac sarcoidosis is well known in case reports, the true incidence of sudden death in cardiac sarcoidosis is unknown. In a clinicopathologic study by Silverman et al,5 seven sudden deaths were noted in 23 patients with cardiac sarcoidosis (7 of 23, 30%). However, this incidence is reported among patients who died of sarcoidosis, thereby inevitably overestimating the true incidence of sudden death.
Studies on the risk stratification in patients with cardiac sarcoidosis have been carried out using various modalities. The presence of DEs in CMR,48, 49, 50 perfusion defect and abnormal FDG uptake,51 inducible sustained VT in programmed electrical stimulation,52 and RV involvement53, 54 have been evaluated as possible risk stratification factors. However, it is still too early to recommend any of these modalities for the decision‐making on ICD implantation. At present, we rely on the major guidelines12, 55 for ICD. In these guidelines, implantation is recommended in patients with a history of cardiac arrest and those with LVEF ≤35%. In our institution, we have divergence of opinion regarding ICD use with a low EF of ≤35%. In patients who are candidates for pacemaker insertion or with unexplained syncope, ICD rather than pacemaker insertion might be a better option.
CONFLICTS OF INTEREST
The authors declare no potential conflict of interests.
Sohn D‐W, Park J‐B, Lee S‐P, Kim H‐K, Kim Y‐J. Viewpoints in the diagnosis and treatment of cardiac sarcoidosis: Proposed modification of current guidelines. Clin Cardiol. 2018;41:1386–1394. 10.1002/clc.23060
REFERENCES
- 1. Iannuzzi MC, Rybicki BA, Teirstein AS. Sarcoidosis. N Engl J Med. 2007;357(21):2153‐2165. [DOI] [PubMed] [Google Scholar]
- 2. Valeyre D, Prasse A, Nunes H, Uzunhan Y, Brillet PY, Muller‐Quernheim J. Sarcoidosis. Lancet. 2014;383(9923):1155‐1167. [DOI] [PubMed] [Google Scholar]
- 3. Lofgren S. Primary pulmonary sarcoidosis. I. Early signs and symptoms. Acta Med Scand. 1953;145(6):424‐431. [PubMed] [Google Scholar]
- 4. Ohta H, Tazawa R, Nakamura A, et al. Acute‐onset sarcoidosis with erythema nodosum and polyarthralgia (Lofgren's syndrome) in Japan: a case report and a review of the literature. Intern Med. 2006;45(9):659‐662. [DOI] [PubMed] [Google Scholar]
- 5. Silverman KJ, Hutchins GM, Bulkley BH. Cardiac sarcoid: a clinicopathologic study of 84 unselected patients with systemic sarcoidosis. Circulation. 1978;58(6):1204‐1211. [DOI] [PubMed] [Google Scholar]
- 6. Johns CJ, Michele TM. The clinical management of sarcoidosis. A 50‐year experience at the Johns Hopkins Hospital. Medicine (Baltimore). 1999;78(2):65‐111. [DOI] [PubMed] [Google Scholar]
- 7. Kandolin R, Lehtonen J, Airaksinen J, et al. Cardiac sarcoidosis: epidemiology, characteristics, and outcome over 25 years in a nationwide study. Circulation. 2015;131(7):624‐632. [DOI] [PubMed] [Google Scholar]
- 8. Uemura A, Morimoto S, Hiramitsu S, Kato Y, Ito T, Hishida H. Histologic diagnostic rate of cardiac sarcoidosis: evaluation of endomyocardial biopsies. Am Heart J. 1999;138(2 Pt 1):299‐302. [DOI] [PubMed] [Google Scholar]
- 9. Cooper LT, Baughman KL, Feldman AM, et al. American Heart Association, American College of Cardiology, European Society of Cardiology The role of endomyocardial biopsy in the management of cardiovascular disease: a scientific statement from the American Heart Association, the American College of Cardiology, and the European Society of Cardiology. Circulation. 2007;116(19):2216‐2233. [DOI] [PubMed] [Google Scholar]
- 10. Hiraga H, Yuwai K, Hiroe M. Guideline for Diagnosis of Cardiac Sarcoidosis: Study Report on Diffuese Pulmonary Disease from the Japanese Ministry of Health and Welfare (in Japanese). Tokyo, Japan: Japanese Ministry of Health and Welfare; 1993:23‐24. [Google Scholar]
- 11. Diagnostic standard and guidelines for sarcoidosis. Japanese J Sarcoidosis Granulomatous Diord. 2007;27:89‐102. [Google Scholar]
- 12. Birnie DH, Sauer WH, Bogun F, et al. HRS expert consensus statement on the diagnosis and management of arrhythmias associated with cardiac sarcoidosis. Heart Rhythm. 2014;11(7):1305‐1323. [DOI] [PubMed] [Google Scholar]
- 13. Kandolin R, Lehtonen J, Graner M, et al. Diagnosing isolated cardiac sarcoidosis. J Intern Med. 2011;270(5):461‐468. [DOI] [PubMed] [Google Scholar]
- 14. Isobe M, Tezuka D. Isolated cardiac sarcoidosis: clinical characteristics, diagnosis and treatment. Int J Cardiol. 2015;182:132‐140. [DOI] [PubMed] [Google Scholar]
- 15. Liang JJ, Hebl VB, DeSimone CV, et al. Electrogram guidance: a method to increase the precision and diagnostic yield of endomyocardial biopsy for suspected cardiac sarcoidosis and myocarditis. JACC Heart Fail. 2014;2(5):466‐473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Nery PB, Keren A, Healey J, Leug E, Beanlands RS, Birnie DH. Isolated cardiac sarcoidosis: establishing the diagnosis with electroanatomic mapping‐guided endomyocardial biopsy. Can J Cardiol. 2013;29(8):1015 e1011‐1015 e1013. [DOI] [PubMed] [Google Scholar]
- 17. Yazaki Y, Isobe M, Hayasaka M, Tanaka M, Fujii T, Sekiguchi M. Cardiac sarcoidosis mimicking hypertrophic cardiomyopathy: clinical utility of radionuclide imaging for differential diagnosis. Jpn Circ J. 1998;62(6):465‐468. [DOI] [PubMed] [Google Scholar]
- 18. Matsumori A, Hara M, Nagai S, et al. Hypertrophic cardiomyopathy as a manifestation of cardiac sarcoidosis. Jpn Circ J. 2000;64(9):679‐683. [DOI] [PubMed] [Google Scholar]
- 19. Uemura A, Morimoto S, Kato Y, et al. Relationship between basal thinning of the interventricular septum and atrioventricular block in patients with cardiac sarcoidosis. Sarcoidosis Vasc Diffuse Lung Dis. 2005;22(1):63‐65. [DOI] [PubMed] [Google Scholar]
- 20. Nagano N, Nagai T, Sugano Y, et al. Association between basal thinning of interventricular septum and adverse long‐term clinical outcomes in patients with cardiac sarcoidosis. Circ J. 2015;79(7):1601‐1608. [DOI] [PubMed] [Google Scholar]
- 21. Vasaiwala SC, Finn C, Delpriore J, et al. Prospective study of cardiac sarcoid mimicking arrhythmogenic right ventricular dysplasia. J Cardiovasc Electrophysiol. 2009;20(5):473‐476. [DOI] [PubMed] [Google Scholar]
- 22. Dechering DG, Kochhauser S, Wasmer K, et al. Electrophysiological characteristics of ventricular tachyarrhythmias in cardiac sarcoidosis versus arrhythmogenic right ventricular cardiomyopathy. Heart Rhythm. 2013;10(2):158‐164. [DOI] [PubMed] [Google Scholar]
- 23. Mahrholdt H, Wagner A, Judd RM, Sechtem U, Kim RJ. Delayed enhancement cardiovascular magnetic resonance assessment of non‐ischaemic cardiomyopathies. Eur Heart J. 2005;26(15):1461‐1474. [DOI] [PubMed] [Google Scholar]
- 24. Bluemke DA. MRI of nonischemic cardiomyopathy. Am J Roentgenol. 2010;195(4):935‐940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Patel MR, Cawley PJ, Heitner JF, et al. Detection of myocardial damage in patients with sarcoidosis. Circulation. 2009;120(20):1969‐1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Mavrogeni S, Apostolou D, Argyriou P, et al. T1 and T2 mapping in cardiology: “mapping the obscure object of desire”. Cardiology. 2017;138(4):207‐217. [DOI] [PubMed] [Google Scholar]
- 27. Puntmann VO, Isted A, Hinojar R, Foote L, Carr‐White G, Nagel E. T1 and T2 mapping in recognition of early cardiac involvement in systemic sarcoidosis. Radiology. 2017;285(1):63‐72. [DOI] [PubMed] [Google Scholar]
- 28. Hung MY, Hung MJ, Cheng CW. Use of gallium 67 scintigraphy to differentiate acute myocarditis from acute myocardial infarction. Tex Heart Inst J. 2007;34(3):305‐309. [PMC free article] [PubMed] [Google Scholar]
- 29. Oka S, Umetani K, Harama T, et al. Cardiac sarcoidosis presenting as acute progressive heart failure with abdominal lymphadenopathy. Intern Med. 2018;57(4):511‐515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Dubrey SW, Bell A, Mittal TK. Sarcoid heart disease. Postgrad Med J. 2007;83(984):618‐623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Takaya Y, Kusano KF, Nakamura K, Ito H. Comparison of outcomes in patients with probable versus definite cardiac sarcoidosis. Am J Cardiol. 2015;115(9):1293‐1297. [DOI] [PubMed] [Google Scholar]
- 32. Kandolin R, Lehtonen J, Airaksinen J, et al. Usefulness of cardiac troponins as markers of early treatment response in cardiac sarcoidosis. Am J Cardiol. 2015;116(6):960‐964. [DOI] [PubMed] [Google Scholar]
- 33. Tanada Y, Sato Y, Sawa T, Fujiwara H, Takatsu Y. Serial measurement of high‐sensitivity cardiac troponin I and N‐terminal proB‐type natriuretic peptide in a patient presenting with cardiac sarcoidosis. Intern Med. 2012;51(24):3379‐3381. [DOI] [PubMed] [Google Scholar]
- 34. Baba Y, Kubo T, Kitaoka H, et al. Usefulness of high‐sensitive cardiac troponin T for evaluating the activity of cardiac sarcoidosis. Int Heart J. 2012;53(5):287‐292. [DOI] [PubMed] [Google Scholar]
- 35. Yazaki Y, Isobe M, Hiroe M, et al. Central Japan Heart Study Group Prognostic determinants of long‐term survival in Japanese patients with cardiac sarcoidosis treated with prednisone. Am J Cardiol. 2001;88(9):1006‐1010. [DOI] [PubMed] [Google Scholar]
- 36. Nagai T, Nagano N, Sugano Y, et al. Effect of corticosteroid therapy on long‐term clinical outcome and left ventricular function in patients with cardiac sarcoidosis. Circ J. 2015;79(7):1593‐1600. [DOI] [PubMed] [Google Scholar]
- 37. Doughan AR, Williams BR. Cardiac sarcoidosis. Heart. 2006;92(2):282‐288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Chapelon‐Abric C, de Zuttere D, Duhaut P, et al. Cardiac sarcoidosis: a retrospective study of 41 cases. Medicine (Baltimore). 2004;83(6):315‐334. [DOI] [PubMed] [Google Scholar]
- 39. Birnie DH, Nery PB, Ha AC, Beanlands RS. Cardiac sarcoidosis. J Am Coll Cardiol. 2016;68(4):411‐421. [DOI] [PubMed] [Google Scholar]
- 40. Statement on sarcoidosis. Joint Statement of the American Thoracic Society (ATS), the European Respiratory Society (ERS) and the World Association of Sarcoidosis and Other Granulomatous Disorders (WASOG) adopted by the ATS Board of Directors and by the ERS Executive Committee, February 1999. Am J Respir Crit Care Med. 1999;160(2):736‐755. [DOI] [PubMed] [Google Scholar]
- 41. Sadek MM, Yung D, Birnie DH, Beanlands RS, Nery PB. Corticosteroid therapy for cardiac sarcoidosis: a systematic review. Can J Cardiol. 2013;29(9):1034‐1041. [DOI] [PubMed] [Google Scholar]
- 42. Futamatsu H, Suzuki J, Adachi S, et al. Utility of gallium‐67 scintigraphy for evaluation of cardiac sarcoidosis with ventricular tachycardia. Int J Cardiovasc Imaging. 2006;22(3–4):443‐448. [DOI] [PubMed] [Google Scholar]
- 43. Winters SL, Cohen M, Greenberg S, et al. Sustained ventricular tachycardia associated with sarcoidosis: assessment of the underlying cardiac anatomy and the prospective utility of programmed ventricular stimulation, drug therapy and an implantable antitachycardia device. J Am Coll Cardiol. 1991;18(4):937‐943. [DOI] [PubMed] [Google Scholar]
- 44. Furushima H, Chinushi M, Sugiura H, Kasai H, Washizuka T, Aizawa Y. Ventricular tachyarrhythmia associated with cardiac sarcoidosis: its mechanisms and outcome. Clin Cardiol. 2004;27(4):217‐222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Jefic D, Joel B, Good E, et al. Role of radiofrequency catheter ablation of ventricular tachycardia in cardiac sarcoidosis: report from a multicenter registry. Heart Rhythm. 2009;6(2):189‐195. [DOI] [PubMed] [Google Scholar]
- 46. Koplan BA, Soejima K, Baughman K, Epstein LM, Stevenson WG. Refractory ventricular tachycardia secondary to cardiac sarcoid: electrophysiologic characteristics, mapping, and ablation. Heart Rhythm. 2006;3(8):924‐929. [DOI] [PubMed] [Google Scholar]
- 47. Muser D, Santangeli P, Pathak RK, et al. Long‐term outcomes of catheter ablation of ventricular tachycardia in patients with cardiac sarcoidosis. Circ Arrhythm Electrophysiol. 2016;9(8):e004333. [DOI] [PubMed] [Google Scholar]
- 48. Vignaux O, Dhote R, Duboc D, et al. Detection of myocardial involvement in patients with sarcoidosis applying T2‐weighted, contrast‐enhanced, and cine magnetic resonance imaging: initial results of a prospective study. J Comput Assist Tomogr. 2002;26(5):762‐767. [DOI] [PubMed] [Google Scholar]
- 49. Murtagh G, Laffin LJ, Beshai JF, et al. Prognosis of myocardial damage in sarcoidosis patients with preserved left ventricular ejection fraction: risk stratification using cardiovascular magnetic resonance. Circ Cardiovasc Imaging. 2016;9(1):e003738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Ekstrom K, Lehtonen J, Hanninen H, Kandolin R, Kivisto S, Kupari M. Magnetic resonance imaging as a predictor of survival free of life‐threatening arrhythmias and transplantation in cardiac sarcoidosis. J Am Heart Assoc. 2016;5(5):e003040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Blankstein R, Osborne M, Naya M, et al. Cardiac positron emission tomography enhances prognostic assessments of patients with suspected cardiac sarcoidosis. J Am Coll Cardiol. 2014;63(4):329‐336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Mehta D, Mori N, Goldbarg SH, Lubitz S, Wisnivesky JP, Teirstein A. Primary prevention of sudden cardiac death in silent cardiac sarcoidosis: role of programmed ventricular stimulation. Circ Arrhythm Electrophysiol. 2011;4(1):43‐48. [DOI] [PubMed] [Google Scholar]
- 53. Crawford T, Mueller G, Sarsam S, et al. Magnetic resonance imaging for identifying patients with cardiac sarcoidosis and preserved or mildly reduced left ventricular function at risk of ventricular arrhythmias. Circ Arrhythm Electrophysiol. 2014;7(6):1109‐1115. [DOI] [PubMed] [Google Scholar]
- 54. Smedema JP, van Geuns RJ, Ector J, Heidbuchel H, Ainslie G, Crijns H. Right ventricular involvement and the extent of left ventricular enhancement with magnetic resonance predict adverse outcome in pulmonary sarcoidosis. ESC Heart Fail. 2017;4:535‐544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. SM A‐K, Stevenson WG, Ackerman MJ, et al. 2017 AHA/ACC/HRS guideline for Management of Patients with Ventricular Arrhythmias and the Prevention of Sudden Cardiac Death: a report of the American College of Cardiology/American Heart Association task force on clinical practice guidelines and the Heart Rhythm Society. J Am Coll Cardiol. 2017;S0735‐1097(17)41306‐4 [Epub ahead of print]. [Google Scholar]


