Abstract
Background
Patients with nonischemic cardiomyopathy (NICM) reportedly have low incidence of appropriate shocks from wearable cardioverter‐defibrillators (WCDs). A recent study questions the benefit from primary preventive implantation of implantable cardioverter‐defibrillators in NICM. We therefore analyzed a subgroup of patients with NICM from the PROLONG study.
Hypothesis
Patients with newly diagnosed NICM show a risk for ventricular tachyarrhythmia.
Methods
The PROLONG study included 167 patients with newly diagnosed heart failure and left ventricular ejection fraction (LVEF) ≤35% with a WCD. Patients with NICM were identified and included in this analysis.
Results
117 patients presented with NICM. Sixty‐five (55%) were male; mean age was 51 ± 15 years. Mean LVEF at diagnosis was 23% ± 7%. Mean follow‐up was 11 ± 10 months. Mean WCD wear time was 101 ± 82 days; mean wear time per day was 21.4 ± 4.5 hours. Overall, 12 ventricular arrhythmias occurred in 10 (9%) patients (6 DCM, 4 PPCM). Nine appropriate WCD shocks for hemodynamically unstable ventricular tachycardia/fibrillation in 8 (7%) patients were observed. Two patients presented sustained hemodynamically stable ventricular tachycardia for >30 minutes detected by the WCD, but withheld WCD therapy.
Conclusions
Patients with newly diagnosed NICM and LVEF ≤35% show an elevated risk of ventricular tachycardia/fibrillation during initiation and optimization of heart failure therapy. To prevent sudden cardiac death, WCD should be considered in patients with newly diagnosed NICM with severely reduced LVEF.
Keywords: Sudden Cardiac Death, Ventricular Arrhythmia, Nonischemic Cardiomyopathy, Wearable Cardioverter‐Defibrillator
1. INTRODUCTION
Patients with nonischemic cardiomyopathy (NICM) were reported to have a low incidence of appropriate wearable cardioverter‐defibrillator (WCD) shocks in previous studies,1, 2 and utility of the WCD in those patients has been impeached.3 According to current guidelines,4, 5 patients with newly diagnosed heart failure (HF) and reduced left ventricular ejection fraction (LVEF) may be temporarily protected from sudden cardiac death (SCD) by the WCD.6 Even in the chronic phase of NICM, benefit from implantable cardioverter‐defibrillators (ICDs) has become a matter of debate after publication of a recent ICD study.7
In the recently published Prolongation of Reverse Remodeling Period to Avoid Untimely ICD Implantation in Newly Diagnosed Heart Failure Using the Wearable Cardioverter/Defibrillator (PROLONG) study on patients with newly diagnosed HF with LVEF ≤35%, we could demonstrate an improvement of LVEF to >35% in 33% of patients during prolonged optimization of HF therapy protected by the WCD beyond a 3‐month waiting period.8
We now analyze a subgroup of patients from the PROLONG study with respect to the occurrence of ventricular tachyarrhythmias in the early phase of NICM with a severely reduced LVEF.
1.1. Methods
The PROLONG study included patients being prescribed with a WCD at Hannover Medical School between June 2012 and January 2016. The detailed study protocol and results have been published elsewhere.8 The study complies with the Declaration of Helsinki and was approved by the local ethics committee. Briefly, 167 patients with newly diagnosed HF and a LVEF ≤35% wearing the WCD were included in the PROLONG study. After diagnosis of HF, patients and treating physicians/cardiologists were instructed to optimize HF medication according to current guidelines.9 All patients received a WCD (LifeVest; Zoll, Pittsburgh, PA) after diagnosis for 3 months and re‐evaluation of LVEF was scheduled. Prolongation of the WCD period was considered if one of the following was present: (1) LVEF at visit 30% to 35%; (2) increase in LVEF of ≥5% compared with the last visit; (3) nonoptimized HF medication.
In this substudy, all patients with NICM were identified from the PROLONG cohort and included in the analysis. Baseline characteristics and follow‐up data at 3 months and last available follow‐up were obtained. Ventricular arrhythmias detected by the WCD during wearing period were analyzed. All patients were prescribed a WCD (LifeVest, Zoll). WCD data were collected via the remote monitoring platform of the manufacturer (LifeVest Network, Zoll).
1.2. Statistical Analysis
Data are presented as mean ± SD for continuous variables or as number of cases and percentages for categorical variables. For comparison of continuous variables over time, a paired t test was performed. Categorical variables were compared using a χ2 test. A P value <0.05 was considered statistically significant. All statistical analyses were performed using SPSS Statistics version 24 (IBM Corp., Armonk, NY).
2. RESULTS
One hundred seventeen patients with NICM were identified. Ninety‐one patients (78%) had dilative cardiomyopathy (DCM), 20 patients (17%) had peripartum cardiomyopathy (PPCM), and 6 patients (5%) had myocarditis. Baseline characteristics are shown in Table 1.
Table 1.
Baseline characteristics of all patients (n = 117) and subentities of nonischemic cardiomyopathy
| All, n = 117 | DCM, n = 91 | PPCM, n = 20 | Myocarditis, n = 6 | |
|---|---|---|---|---|
| Male sex | 64 (56) | 62 (68) | 0 (0) | 3 (50) |
| Age, y | 51 ± 15 (95% CI: 49–54) | 55 ± 14 (95% CI: 52–58) | 33 ± 4 (95% CI: 31–35) | 52 ± 11 (95% CI: 43–61) |
| LVEF at diagnosis, % | 23 ± 7 (95% CI: 22–24) | 24 ± 7 (95% CI: 23–25) | 21 ± 7 (95% CI: 18–24) | 16 ± 4 (95% CI: 12–19) |
| NYHA class at diagnosis | 2.8 ± 0.7 (95% CI: 2.7‐2.9) | 2.7 ± 0.7 (95% CI: 2.6‐2.8) | 3.4 ± 0.7 (95% CI: 3.1‐3.6) | 3 ± 0 (NA) |
| Rhythm at diagnosis | ||||
| Sinus | 102 (87) | 77 (85) | 20 (100) | 5 (83) |
| AF | 15 (13) | 14 (15) | 0 | 1 (17) |
| HR at diagnosis, bpm | 82 ± 18 (95% CI: 79–85) | 82 ± 18 (95% CI: 79–86) | 83 ± 19 (95% CI: 74–91) | 73 ± 8 (95% CI: 66–79) |
| QRS duration at diagnosis, ms | 113 ± 27 (95% CI: 108–117) | 115 ± 26 (95% CI: 109–120) | 100 ± 26 (95% CI: 88–111) | 124 ± 26 (95% CI: 104–145) |
| NT‐proBNP at diagnosis, ng/L | 6722 ± 9351 (95% CI: 5027–8416) | 7252 ± 9787 (95% CI: 5242–9263) | 5693 ± 8370 (95% CI: 2025–9361) | 2796 ± 2422 (95% CI: 858–4734) |
| Medications | ||||
| β‐Blocker | 114 (97) | 89 (98) | 19 (95) | 6 (100) |
| ACEI/ARB | 114 (97) | 88 (97) | 20 (100) | 6 (100) |
| MRA | 107 (91) | 82 (90) | 19 (95) | 6 (100) |
| Diuretics | 97 (83) | 73 (80) | 18 (90) | 6 (100) |
| Ivabradine | 33 (28) | 17 (19) | 15 (75) | 1 (17) |
| Digitalis | 15 (13) | 14 (15) | 0 (0) | 1 (17) |
Abbreviations: ACEI, angiotensin‐converting enzyme inhibitor; AF, atrial fibrillation; ARB, angiotensin receptor blocker; CI, confidence interval; DCM, dilative cardiomyopathy; HR, heart rhythm; LVEF, left ventricular ejection fraction; MRA, mineralocorticoid receptor antagonist; NA, not applicable; NT‐proBNP, N‐terminal pro b‐type natriuretic peptide; NYHA, New York Heart Association; PPCM, peripartum cardiomyopathy; QRS, QRS interval; SD, standard deviation.
Data are presented as n (%) or mean ± SD.
2.1. Follow‐up
Mean follow‐up was 11 ± 10 months (95% confidence interval [CI]: 10–13 months). Three (3%) patients were lost to follow‐up. Mean LVEF at last follow‐up was 40% ± 11% (95% CI: 38%‐42%). Difference between LVEF at diagnosis and LVEF at last follow‐up was highly significant (P < 0.0001). Mean NYHA functional class at last follow‐up was 1.8 ± 0.6 (95% CI: 1.7‐1.9). Five patients (4%) died during follow‐up; mode of death was terminal heart failure in 2 (2%), intracranial hemorrhage in 1 (1%), end‐stage kidney disease in 1 (1%), and septicemia in 1 (1%) patient. Two (2%) patients received a left ventricular assist device.
At last follow‐up, 37 (32%) patients had received an ICD. Eight (7%) patients refused an ICD despite presentation of an accepted indication according to current guidelines. The ICD systems were single chamber in 16 (14%) patients, dual chamber in 5 (4%) patients, cardiac resynchronization therapy defibrillator (CRT‐D) in 14 (12%) patients, and VDD mode in 2 (2%) patients.
2.2. WCD data
Cumulative WCD wearing time adds up to >31 years (11 512 days). Mean WCD wear time was 101 ± 82 days (95% CI: 86–116 days); mean WCD wear time per day was 21.4 ± 4.5 hours (95% CI: 20.5‐22.3 hours).
2.3. Arrhythmias
Overall, 12 ventricular arrhythmias occurred in 10 (9%) patients (6 DCM, 4 PPCM) during the WCD wearing period (Figure 1, Table 2). The extrapolated event rate was 38.7 ventricular tachyarrhythmias per 100 person‐years. Mean time from diagnosis to ventricular arrhythmia was 68 ± 42 days (range, 28–161 days; median, 60 days; 95% CI: 42–94 days; Figure 2). Nine appropriate WCD shocks for ventricular tachycardia/fibrillation (VT/VF) were observed in 8 patients (5 DCM, 3 PPCM). All WCD shocks were efficient with first shock. Two more patients presented hemodynamically stable sustained ventricular tachycardia for >30 minutes detected by the WCD, but withheld WCD therapy by pressing the response buttons. No asystoles or inappropriate therapies occurred.
Figure 1.
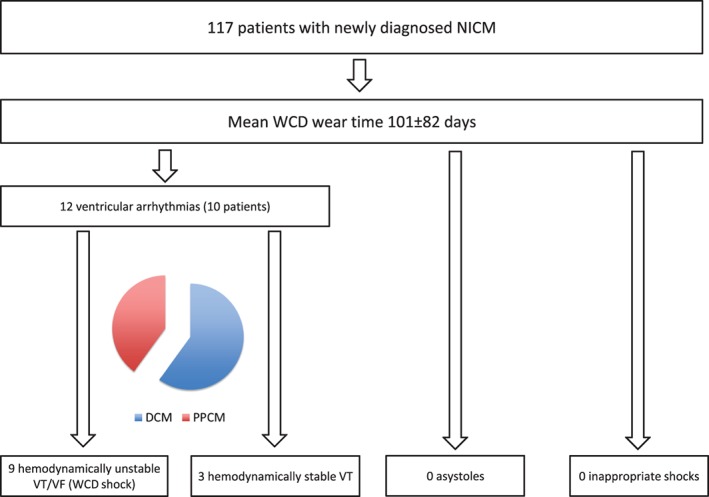
Arrhythmic events during WCD wearing period in 117 patients with newly diagnosed NICM. Abbreviations: DCM, dilative cardiomyopathy; NICM, nonischemic cardiomyopathy; PPCM, peripartum cardiomyopathy; VF, ventricular fibrillation; VT, ventricular tachycardia; WCD, wearable cardioverter‐defibrillator.
Table 2.
Characteristics of patients with ventricular tachyarrhythmias (n = 10)
| n = 10 | |
|---|---|
| Male sex | 2 (20) |
| Age, y | 48 ± 16 (95% CI: 38–58) |
| Diagnosis | |
| NICM | 6 (60) |
| PPCM | 4 (40) |
| LVEF at diagnosis, % | 21.2 ± 6.1 (95% CI: 17.4‐25.0) |
| NYHA class at baseline | 2.9 ± 0.7 (95% CI: 2.5 ± 3.3) |
| Rhythm at baseline | |
| Sinus | 8 (80) |
| AF | 2 (20) |
| HR at baseline, bpm | 85 ± 20 (95% CI: 73–98) |
| QRS duration at diagnosis, ms | 116 ± 23 (95% CI: 102–130) |
| NT‐proBNP at diagnosis, ng/L | 6339 ± 6747 (95% CI: 2158–10 521) |
| Medications at discharge | |
| β‐Blocker | 10 (100) |
| ACEI/ARB | 10 (100) |
| MRA | 9 (90) |
| Diuretics | 8 (80) |
| Ivabradine | 3 (30) |
| Digitalis | 2 (20) |
Abbreviations: ACEI, angiotensin‐converting enzyme inhibitor; AF, atrial fibrillation; ARB, angiotensin receptor blocker; CI, confidence interval; HR, heart rhythm; LVEF, left ventricular ejection fraction; MRA, mineralocorticoid receptor antagonist; NICM, nonischemic cardiomyopathy; NT‐proBNP, N‐terminal pro b‐type natriuretic peptide; NYHA, New York Heart Association; PPCM, peripartum cardiomyopathy; QRS, QRS interval; SD, standard deviation.
Data are presented as n (%) or mean ± SD.
Figure 2.
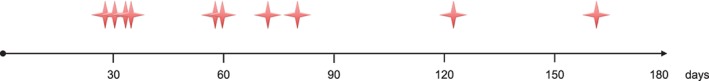
Timing of ventricular tachyarrhythmias during WCD period. Abbreviations: WCD, wearable cardioverter‐defibrillator.
Patients with and without VT/VF events did not show significant differences in etiology, age, NYHA functional class, LVEF, heart rate, QRS duration, or N‐terminal pro–b‐type natriuretic peptide level at diagnosis. Patients having presented sustained VT/VF received a secondary prophylactic ICD.
3. DISCUSSION
In our cohort of patients with newly diagnosed NICM with LVEF ≤35%, we found a relevant amount of life‐threatening ventricular tachyarrhythmias. In a relatively small cohort of only 117 patients, 10 patients showed 12 episodes of VT or VF.
These findings contrast with a previously published study that did not show any WCD shock in 254 patients with newly diagnosed NICM.3 However, the incidence of zero ventricular tachyarrhythmias in this cohort is not supported by the majority of WCD studies in NICM patients1, 2, 10, 11 and might be partly explained by a shorter WCD wearing time of 71 days (median).3 Another recent study on patients with NICM wearing the WCD already reported a relevant risk for ventricular tachyarrhythmias in newly diagnosed HF.10 However, the studied patient population only consisted of patients with alcoholic cardiomyopathy, thereby representing a special subentity of NICM. Furthermore, overall prognosis of patients with long‐term alcohol consumption is poor. Compliance is a problem in these patients, as shown by a median wear time of only 51 days and a median wear time of 18.0 hours per day.10 In our study, we had mainly patients with nonalcoholic cardiomyopathy and with remarkably high wear time.
Arrhythmia burden is supposed to be elevated during the early phase of reverse ventricular remodeling.12 This assumption is supported by our study. Possibly, after optimization of HF therapies, the risk for lethal arrhythmias might decrease and thereby mitigate the effect of long‐term primary preventive ICD therapy, explaining the findings of the Danish ICD Study in Patients With Dilated Cardiomyopathy (DANISH) trial.7 A special situation of highly vulnerable patients during the early phase until recovery may be found in PPCM. In acute PPCM, patients often present with severely reduced LVEF but may quickly recover in LVEF in the first months.13, 14 However, patients show an elevated risk for life‐threatening arrhythmias during the first 3 to 6 months.11, 17
In the current trial, we found an incidence of ventricular tachyarrhythmias in the early stage of NICM of 38.7 per 100 person‐years. In the ICD group in DANISH, providing the most actual data on VT/VF in the chronic phase of NICM, the incidence was reported to be 5.9 per 100 person‐years (161 events of antitachycardia pacing or shock in cumulative 2732.5 person‐years).7 Baseline LVEF in both studies was comparable. Thus, the occurrence of VT/VF was more than 6‐fold higher in our study in patients with newly diagnosed NICM and nonoptimized medical therapy compared with patients in the chronic phase of NICM.
According to the results of the Intervention in Myocarditis and Acute Cardiomyopathy‐2 (IMAC‐2) study, early implantation of an ICD did not improve survival in patients with NICM.15 The main reason seems to be the large potential of LVEF recovery after newly diagnosed NICM. During follow‐up of the IMAC‐2 study, mean LVEF increased from 24% at diagnosis to 42% at 6‐month follow‐up in the nondevice group.15 However, physicians may implant ICDs too early.16 One reason might be the fear of SCD during optimization of medical therapy. Implantation of an ICD is only indicated in patients with optimal medical therapy over a period of ≥3 months. Considering this, ICD implantation 3 months after diagnosis has to be too early in nearly all patients, because patients would be assumed to be on optimal pharmacological therapy already at the moment of diagnosis.
Relevance of sudden arrhythmic death in patients with chronic NICM has been called into question in patients with optimal medical therapy. The recently published DANISH study did not show a significant difference in mortality in patients with NICM between patients receiving an ICD and patients without an ICD.7 Nevertheless, there was a highly significant reduction of SCD in the ICD group (hazard ratio: 0.5), showing that there is indeed a relevant amount of arrhythmias necessitating ICD therapy.7 The DANISH trial included patients with NICM and an LVEF ≤35%, but it required optimized medical treatment with dosages at target levels as well. Therefore, the study population of the DANISH trial does not correspond to our study population, because we only enrolled patients with newly diagnosed NICM not yet on optimal medical treatment.
Furthermore, the age of patients with NICM appears to be of major importance. In a subgroup analysis of DANISH, patients age <59 years appeared to benefit from the ICD in terms of total mortality. Mean age in our cohort was 51, maybe contributing to the benefit of SCD prevention. DANISH underlines that it is important to establish optimal HF medication before decision for permanent ICD implantation is taken. Optimization of HF medication and reverse ventricular remodeling takes time, which can be securely provided by the WCD as presented in the PROLONG study.8
3.1. Study limitations
This is a retrospective analysis only including patients having received a WCD at our center. It may therefore be subject to selection and reporting bias. Nevertheless, in a small but well‐characterized cohort of patients, we found a relevant amount of life‐threatening arrhythmias, thereby objecting to previous WCD studies. Our results therefore stimulate further research in evaluating the role of the WCD after diagnosis of NICM. The results of the present study question previous publications on low risk after diagnosis of NICM.
4. CONCLUSION
Patients with newly diagnosed NICM and LVEF ≤35% show an elevated risk for life‐threatening ventricular arrhythmias during the early phase of optimization of HF therapy when ICD implantation is not indicated. There were no significant predictors for VT/VF events in baseline parameters. According to the results of the present study, WCD should be considered in patients with newly diagnosed NICM with severely reduced LVEF.
4.1. Conflicts of interest
DD received lecture honoraria and travel support from Zoll. CV received lecture honoraria, travel support, and a research grant from Zoll. The authors declare no other potential conflicts of interest.
Duncker D, König T, Hohmann S, Bauersachs J and Veltmann C. Ventricular arrhythmias in patients with newly diagnosed nonischemic cardiomyopathy: Insights from the PROLONG study. Clin Cardiol. 2017;40:586–590. 10.1002/clc.22706
REFERENCES
- 1. Wäßnig NK, Günther M, Quick S, et al. Experience with the wearable cardioverter‐defibrillator in patients at high risk for sudden cardiac death. Circulation. 2016;134:635–643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Kutyifa V, Moss AJ, Klein H, et al. Use of the wearable cardioverter‐defibrillator in high‐risk cardiac patients: data from the Prospective Registry of Patients Using the Wearable Cardioverter Defibrillator (WEARIT‐II Registry). Circulation. 2015;132:1613–1619. [DOI] [PubMed] [Google Scholar]
- 3. Singh M, Wang NC, Jain S, et al. Utility of the wearable cardioverter‐defibrillator in patients with newly diagnosed cardiomyopathy: a decade‐long single‐center experience. J Am Coll Cardiol. 2015;66:2607–2613. [DOI] [PubMed] [Google Scholar]
- 4. Ponikowski P, Voors AA, Anker SD, et al. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: the Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC). Developed with the special contribution of the Heart Failure Association (HFA) of the ESC [published correction appears in Eur Heart J. 2016;pii:ehw383]. Eur Heart J . 2016;37:2129–2200. [DOI] [PubMed] [Google Scholar]
- 5. Priori SG, Blomström‐Lundqvist C, Mazzanti A, et al. 2015 ESC Guidelines for the management of patients with ventricular arrhythmias and the prevention of sudden cardiac death: the Task Force for the Management of Patients with Ventricular Arrhythmias and the Prevention of Sudden Cardiac Death of the European Society of Cardiology (ESC). Endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC). Eur Heart J . 2015;36:2793–2867. [DOI] [PubMed] [Google Scholar]
- 6. Duncker D, Veltmann C. The wearable cardioverter/defibrillator—toy or tool? J Atr Fibrillation. 2016;8:1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kober L, Thune JJ, Nielsen JC, et al. Defibrillator implantation in patients with nonischemic systolic heart failure. N Engl J Med. 2016;375:1221–1230. [DOI] [PubMed] [Google Scholar]
- 8. Duncker D, König T, Hohmann S, et al. Avoiding Untimely Implantable Cardioverter/Defibrillator Implantation by Intensified Heart Failure Therapy Optimization Supported by the Wearable Cardioverter/Defibrillator—the PROLONG Study. J Am Heart Assoc . 2017. doi: 10.1161/JAHA.116.004512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. McMurray JJ, Adamopoulos S, Anker SD, et al; ESC Committee for Practice Guidelines . ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure 2012: the Task Force for the Diagnosis and Treatment of Acute and Chronic Heart Failure 2012 of the European Society of Cardiology. Developed in collaboration with the Heart Failure Association (HFA) of the ESC [published correction appears in Eur Heart J. 2013;34:158]. Eur Heart J . 2012;33:1787–1847. [DOI] [PubMed] [Google Scholar]
- 10. Salehi N, Nasiri M, Bianco NR, et al. The wearable cardioverter defibrillator in nonischemic cardiomyopathy: a US national database analysis. Can J Cardiol . 2016;32:1247.e1–1247.e6. [DOI] [PubMed] [Google Scholar]
- 11. Duncker D, Haghikia A, König T, et al. Risk for ventricular fibrillation in peripartum cardiomyopathy with severely reduced left ventricular function—value of the wearable cardioverter/defibrillator. Eur J Heart Fail. 2014;16:1331–1336. [DOI] [PubMed] [Google Scholar]
- 12. Lip GY, Heinzel FR, Gaita F, et al. European Heart Rhythm Association/Heart Failure Association joint consensus document on arrhythmias in heart failure, endorsed by the Heart Rhythm Society and the Asia Pacific Heart Rhythm Society. Europace. 2016;18:12–36. [DOI] [PubMed] [Google Scholar]
- 13. Bauersachs J, Arrigo M, Hilfiker‐Kleiner D, et al. Current management of patients with severe acute peripartum cardiomyopathy: practical guidance from the Heart Failure Association of the European Society of Cardiology Study Group on peripartum cardiomyopathy. Eur J Heart Fail. 2016;18:1096–1105. [DOI] [PubMed] [Google Scholar]
- 14. Haghikia A, Podewski E, Libhaber E, et al. Phenotyping and outcome on contemporary management in a German cohort of patients with peripartum cardiomyopathy. Basic Res Cardiol. 2013;108:366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Sheppard R, Mather PJ, Alexis JD, et al. Implantable cardiac defibrillators and sudden death in recent onset nonischemic cardiomyopathy: results from IMAC2. J Card Fail. 2012;18:675–681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Al‐Khatib SM, Hellkamp A, Curtis J, et al. Non‐evidence‐based ICD implantations in the United States. JAMA. 2011;305:43–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Duncker D, Westenfeld R, Konrad T, Pfeffer T, Correia de Freitas CA, Pfister R, Thomas D, Fürnkranz A, Andrié RP, Napp A, Schmitt J, Karolyi L, Wakili R, Hilfiker‐Kleiner D, Bauersachs J, Veltmann C. Risk for life‐threatening arrhythmia in newly diagnosed peripartum cardiomyopathy with low ejection fraction: a German multi‐centre analysis. Clin Res Cardiol. 2017. Mar 8. doi: 10.1007/s00392-017-1090-5. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]


