Abstract
Background
Little is known about the comparative effectiveness and safety of non–vitamin K antagonist oral anticoagulants (NOAC) compared to warfarin in Chinese atrial fibrillation (AF) patients. Our aim was to compare the ischemic stroke risk reduction and incidence of intracranial hemorrhage (ICH) of warfarin in relation to quality of anticoagulation control (as reflected by time in therapeutic range [TTR]), and to dabigatran and rivaroxaban in a real‐world cohort of Chinese AF patients.
Hypothesis
NOAC, rather than warfarin, is preferred in Chinese AF patients.
Methods
Of 2099 patients studied (73.1 ± 12.3 years, female: 44.6%, CHA2DS2‐VASc 3.7 ± 1.9 and HAS‐BLED 2.0 ± 1.0) with nonvalvular AF, 963 patients (45.9%) were on warfarin (only 16.3% had TTR ≥65%), 669 patients were on rivaroxaban, and 467 patients were on dabigatran.
Results
After a mean follow‐up of 21.7 ± 13.4 months, there were 156 ischemic strokes (annual incidence of 4.10%/year), with the incidence of ischemic stroke being highest in patients on warfarin with TTR <65% (5.24%/year), followed by those on rivaroxaban (3.74%/year), and those on warfarin with TTR ≥65% (3.35%/year), whereas patients on dabigatran had the lowest incidence of ischemic stroke (1.89%/year). The incidence of ICH was lowest in patients on dabigatran (0.39%/year) compared with those on rivaroxaban (0.52%/year) and warfarin, with TTR <65% (0.95%/year) and TTR ≥65% (0.58%/year). Patients on rivaroxaban 20 mg daily had similar ischemic stroke risk (1.93%/year) and ICH risk (0.21%/year) compared to dabigatran.
Conclusions
In Chinese AF patients, the benefits of warfarin therapy for stroke prevention and ICH reduction depend on TTR. Of the treatments compared, dabigatran, as well as rivaroxaban 20 mg daily, was associated with lowest ischemic stroke and ICH rates.
Keywords: Atrial fibrillation, Non‐vitamin K antagonist oral anticoagulants, Ischemic stroke, Intracranial hemorrhage
1. INTRODUCTION
Despite a lower prevalence of atrial fibrillation (AF) among the Chinese population (0.7%–1%) compared with Caucasians (1%–2%),1, 2 the overall disease burden in the Chinese population is much higher due to the huge and proportionally larger, aged population. There were more than 8 million AF patients in mainland China,1 which were 3 times that seen in the United States.
Long‐term oral anticoagulant for stroke prevention is the cornerstone of AF management. For more than 2 decades, warfarin with a target International Normalized Ratio (INR) between 2.0 and 3.0 is the only effective therapy for stroke prevention in AF. Although warfarin therapy effectively reduces ischemic stroke and mortality among patients with AF,3 this therapy has long been grossly underutilized in the Chinese population.4 This is partly related to the high intracranial hemorrhage (ICH) risk inherent to such therapy in the Chinese population,5 and partly to the widely perceived difference in warfarin sensitivity between Chinese and Caucasians leading to poor anticoagulation control, as determined by the time in therapeutic range (TTR).6
The non–vitamin K antagonist oral anticoagulants (NOACs) including dabigatran, rivaroxaban, apixaban, and edoxaban have all been demonstrated in large randomized control trials to be at least as effective as warfarin in stroke reduction but with a lower ICH risk.3 In subanalyses of these major NOAC studies comparing Asian and non‐Asian populations, there are some differences in efficacy and safety profile in Asian and non‐Asian populations.7, 8 In stark contrast to their huge disease burden, Chinese AF patients have been grossly under‐represented in all major NOAC studies.9, 10, 11 For instance, Chinese patients typically constitute only ~5% of the total study populations in these major studies: 5.4% (986 out of 18 133 patients) in the RE‐LY (Randomized Evaluation of Long‐Term Anticoagulation Therapy) trial,9 5.1% (728 out of 14,264 patients) in the ROCKET‐AF (Rivaroxaban Once Daily Oral Direct Factor Xa Inhibition Compared with Vitamin K Antagonism for Prevention of Stroke and Embolism Trial in Atrial Fibrillation) trial,10 and 5.4% (976 out of 18,201 patients) in the ARISTOTLE (Apixaban for Reduction in Stroke and Other Thromboembolic Events in Atrial Fibrillation ) trial.11
Unlike in the United States and other developed countries, where postmarketing surveillance is actively performed for these new agents in the general population and in populations under‐represented in the original trials, real‐world data focusing on the Chinese population are largely missing. Although the gold standard to demonstrate treatment efficacy remains randomized control trials, a real‐world clinical registry that includes patients who are not adequately represented in clinical trials can provide additional information to fill this void in knowledge.
The aim of our study was to compare the efficacy and safety of dabigatran, rivaroxaban, and warfarin in a large contemporary prospective real‐world hospitalized cohort of Chinese AF patients with detailed long‐term follow‐up.
2. METHODS
2.1. Study design
This was an observational study from a hospital‐based AF registry.4, 6, 12, 13 The study protocol was approved by the local institutional review board. Patients diagnosed with AF in Queen Mary Hospital, Hong Kong, from January 2008 to December 2014, were identified via the computerized database of the clinical management system. The public healthcare system in Hong Kong, which takes care of 95% of the total population, remained consistent over the study period. Demographic data, cardiovascular risk factors, and medications were recorded at baseline. This administrative dataset is well validated for the primary coding diagnosis of AF. A diagnosis of AF in the computer‐based clinical management system has a positive predictive value of 99% of AF cases. All hospital admissions, outpatient clinic visits, laboratory results, and radiological images had been recorded in the clinical management system since 1996. Demographic data, cardiovascular risk factors, and medications were recorded at baseline. The index date was defined as the date of the first occurrence of AF. For registration of outcome during follow‐up, a blanking period of 14 days after the index date was applied, as the occurrence of a stroke or ICH within the first few days of diagnosis of AF is most likely related to initial presentation rather than a new event.
Ischemic stroke risk was estimated at baseline using the CHA2DS2‐VASc score (C: congestive heart failure [1 point], H: hypertension [1 point], A2: age 65–74 years [1 point] and age ≥75 years [2 points], D: diabetes mellitus [1 point], S: prior stroke or transient ischemic attack [2 points], VA: vascular disease [1 point]; and Sc: sex category [female] [1 point]) as described in recent guidelines. Likewise, the HAS‐BLED (H: uncontrolled hypertension (systolic blood pressure >160 mm Hg) (1 point), A: abnormal renal function (serum creatinine >200 µmol/L) (1 point) and abnormal liver function (cirrhosis or bilirubin >2 times upper limits of normal or aspartate aminotransferase alanine transaminase alkaline phosphatase >3 times upper limits of normal) (1 point), S: previous stroke (1 point); B: prior major bleeding (1 point), L: labile INR (<60% TTR), E: age >65 years (elderly) (1 point), D: drugs predisposing to bleeding, alcohol (>8 drinks/week) score was calculated at baseline as a measure of bleeding risk. Uncontrolled hypertension was defined as systolic blood pressure >160 mm Hg at baseline and subsequent visit‐to‐visit changes in systolic blood pressure were not taken into account. Similarly, liver disease determined by the derangement in liver biochemistry and renal disease determined by serum creatinine level were only assessed at baseline, and subsequent changes were not taken into account. Patients were excluded if they had CHA2DS2‐VASc <1, significant valvular heart disease including mitral stenosis of any degree and/or prosthetic heart valves, or incomplete clinical and/or follow‐up data. The final analysis included 2099 patients with nonvalvular AF with a CHA2DS2‐VASc of 1 or above, who were classified according to their antithrombotic strategies: warfarin (TTR ≥65% or <65%), dabigatran, and rivaroxaban. According to the center's protocol, INR was measured every 8 weeks and more frequently when INR was not within the therapeutic range. Among patients receiving warfarin therapy, TTR was calculated for each patient using the Rosendaal method, in which INR was assumed to change in a linear manner between measurements, and INR values on the days with no measurement were interpolated. However, INR measurements within the first 6 weeks of warfarin therapy were excluded from this analysis due to the more frequent INR testing and large fluctuation in measurements during initial warfarin adjustment. The percentage of time during which a patient had an INR within 2.0 to 3.0 was taken as the TTR. Patients were then further classified into 2 groups according to TTR, either ≥65% or <65%.
2.1.1. Outcomes, variables, and data source
The primary outcome was a composite of hospital admission with ischemic stroke or ICH, or death during the follow‐up period. The secondary outcome was hospital admission for ischemic stroke and/or ICH. Stroke was defined as a neurological deficit of sudden onset, persisting for >24 hours, corresponding to a vascular territory and not explained by other causes (eg, trauma, infection). ICH comprises intracerebral hemorrhage, subarachnoid hemorrhage, and subdural hemorrhage.14 Neuroimaging evidence, either from computerized tomography or magnetic resonance imaging, was required to confirm the diagnosis of stroke and ICH. Major bleeding was defined as a fatal bleeding event, a hospitalized bleeding event requiring transfusion, or hospitalization with hemorrhage into a critical site (ie, intracranial, intraspinal, intra‐articular, intraocular, pericardial, retroperitoneal, or intramuscular with compartment syndrome). The International Classification of Diseases, Tenth Revision, Clinical Modification codes were used to define these clinical events/outcomes. Data were retrieved from the medical records, and discharge summaries were retrieved from the territory‐wide information network of all public hospitals in Hong Kong.
2.2. Statistical analysis
Data are expressed as mean ± standard derivation for continuous variables and frequency (%) for categorical variables. For continuous variables, an independent sample Student t test was used for intergroup comparisons. Categorical variables were compared using Pearson χ2 test or Fisher exact test if more than 10% of the cells had an expected count <5. Kaplan‐Meier survival analyses with the log‐rank test were carried out, and Cox proportional hazards regression model was used to calculate hazard ratios (HRs) of some predictive factors and their 95% confidence interval (CIs) for the incidence of stroke. Calculations were performed using SPSS version 21.0 software (IBM, Armonk, NY). All tests were 2‐sided, and P values were considered significant if <0.05.
3. RESULTS
A total of 2099 patients (73.1 ± 12.3 years, female: 44.6%) with nonvalvular AF were included in the final analysis (Figure 1A). Table 1 summarizes the clinical characteristics of the study population. The mean CHA2DS2‐VASc score and mean HAS‐BLED score were 3.7 ± 1.9, and 2.0 ± 1.0, respectively. Of this cohort, 963 patients (45.9%) were on warfarin. The mean and median TTR of the warfarin users were 39.7% and 41.0%, respectively. Only 157 out of 963 patients on warfarin achieved good anticoagulation control with TTR ≥65% (16.3%, mean TTR 77.5 ± 9.3%, median TTR: 75.7% [interquartile range: 69.9%–83.7%]). Another 669 patients were taking rivaroxaban (394 [59%] on 20 mg daily and 275 on 15 mg daily [41%]), and 467 patients were taking dabigatran (397 [85%] on 110 mg 2 times a day, 54 [12%] on 75 mg 2 times a day, and 16 [3%] on 150 mg 2 times a day).
Figure 1.
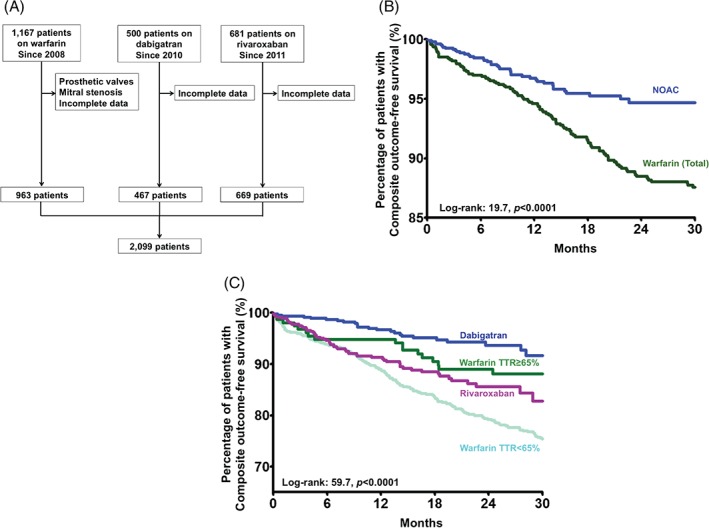
(A) Study population and flowchart of patient selection. (B) Kaplan‐Meier estimates of the composite outcome‐free survival in Chinese AF patients on warfarin and on a NOAC. (C) Kaplan‐Meier estimates of the composite outcome‐free survival in Chinese AF patients on warfarin with TTR ≥65%, patients on warfarin with TTR <65%, patients on rivaroxaban, and patients on dabigatran. Abbreviations: AF, atrial fibrillation; NOAC, non–vitamin K antagonist oral anticoagulant; TTR, time in therapeutic range.
Table 1.
Baseline characteristics
| All, N = 2099 | Warfarin | Rivaroxaban, N = 669 | Dabigatran, N = 467 | P Value1 | P Value2 | ||
|---|---|---|---|---|---|---|---|
| TTR <65%, N = 806 | TTR ≥65%, N = 157 | ||||||
| Mean age, y | 73.1 ± 12.3 | 73.9 ± 13.2 | 71.1 ± 11.7 | 73.3 ± 12.1 | 71.9 ± 11.1 | 0.0383 | 0.05 |
| Female, n (%) | 936 (44.6) | 379 (47.0) | 69 (43.9) | 269 (40.2) | 219 (46.9) | 0.08 | 0.033 |
| HT, n (%) | 1,475 (70.3) | 493 (61.2) | 101 (64.3) | 569 (85.1) | 312 (66.8) | <0.00013 | <0.00013 |
| DM, n (%) | 564 (26.9) | 207 (25.7) | 22 (14.0) | 211 (31.5) | 124 (26.6) | <0.00013 | 0.07 |
| Heart failure, n (%) | 505 (24.1) | 208 (25.8) | 26 (16.6) | 160 (23.9) | 111 (23.8) | 0.125 | 0.954 |
| CAD, n (%) | 312 (14.9) | 298 (24.6) | 25 (22.3) | 53 (7.9) | 26 (5.6) | <0.00013 | 0.125 |
| Stroke/TIA, n (%) | 215 (26.7) | 215 (26.7) | 51 (32.5) | 178 (26.6) | 135 (28.9) | 0.304 | 0.393 |
| Liver disease, n (%) | 281 (13.4) | 189 (23.4) | 30 (19.1) | 46 (6.9) | 16 (3.4) | <0.00013 | 0.0123 |
| Abnormal renal function, n (%) | 24 (1.1) | 16 (2.0) | 1 (0.6) | 3 (0.4) | 4 (0.9) | 0.0353 | 0.387 |
| Prior major bleeding, n (%) | 71 (3.4) | 9 (1.1) | 2 (1.3) | 7 (1.0) | 53 (11.3) | <0.00013 | <0.00013 |
| Drugs and/or alcohol use to bleeding, n (%) | 97 (4.6) | 35 (4.3) | 6 (3.8) | 3 (0.4) | 53 (11.3) | <0.00013 | <0.00013 |
| Mean CHADS2 | 2.4 ± 1.5 | 2.5 ± 1.5 | 2.2 ± 1.5 | 2.5 ± 1.5 | 2.2 ± 1.4 | 0.0043 | 0.0093 |
| Mean CHA2DS2‐VASc | 3.7 ± 1.9 | 3.8 ± 2.0 | 3.4 ± 2.0 | 3.7 ± 1.8 | 3.6 ± 1.9 | 0.163 | 0.231 |
| Mean HAS‐BLED | 2.0 ± 1.0 | 2.1 ± 1.0 | 2.0 ± 1.0 | 2.0 ± 0.9 | 2.0 ± 1.1 | 0.726 | 0.456 |
| Mean TTR | — | 32.3 ± 19.8 | 77.5 ± 9.3 | — | — | — | — |
Abbreviations: CAD, coronary artery disease; CHADS2 score, congestive heart failure, hypertension, age ≥75 years, diabetes, previous stroke; CHA2DS2‐VASc score, C: congestive heart failure (1 point), H: hypertension (1 point), A2: age 65 to 74 years (1 point) and age ≥75 years (2 points), D: diabetes mellitus (1 point), S: prior stroke or transient ischemic attack (2 points), VA: vascular disease (1 point), and Sc: sex category (female) (1 point); DM, diabetes mellitus; HAS‐BLED score, H: uncontrolled hypertension (systolic blood pressure >160 mm Hg) (1 point), A: abnormal renal function (serum creatinine >200 µmol/L) (1 point) and abnormal liver function (cirrhosis or bilirubin >2 times upper limits of normal or aspartate aminotransferase alanine transaminase alkaline phosphatase >3 times upper limits of normal) (1 point), S: previous stroke (1 point); B: prior major bleeding (1 point), L: labile INR (<60% TTR), E: age >65 years (elderly) (1 point), D: drugs predisposing to bleeding, alcohol (>8 drinks/week); HT, hypertension; INR, International Normalized Ratio; TIA, transient ischemic attack; TTR, time in therapeutic range.
Data are expressed as mean ± standard derivation for continuous variables and frequency (%) for categorical variables.
P value for comparison between patients on warfarin with TTR ≥65%, on dabigatran, and on rivaroxaban.
P value for comparison between patients on dabigatran and on rivaroxaban.
P < 0.05.
Compared with patients on warfarin with TTR ≥65% and patients on dabigatran, patients on rivaroxaban were older (73.3 ± 12.1 years vs 71.1 ± 11.7 years and 71.9 ± 11.1 years, P = 0.038), and more likely to have hypertension (85.1% vs 64.3% and 66.8%, P < 0.0001) and diabetes mellitus (31.5% vs 14.0% and 26.6%, P < 0.0001). Patients on warfarin with TTR ≥65% were more likely to have coronary artery disease compared with patients on rivaroxaban and dabigatran (22.3% vs 7.9% and 5.6%, P < 0.0001). The mean CHA2DS2‐VASc and HAS‐BLED scores did not differ significantly between these 3 groups (Table 1).
3.1. Clinical outcomes
After a mean follow‐up of 21.7 ± 13.4 months, a primary end point had occurred for 478 patients (22.8%). The primary outcome had been reached by 387 patients on warfarin therapy (40.2%) compared with 91 patients with a NOAC (8.01%). Figure 1B depicts the Kaplan‐Meier analysis of primary outcomes among patients on warfarin and a NOAC (log‐rank: 19.7, P < 0.0001), and Figure 1C depicts the analysis among patients on warfarin with TTR <65%, warfarin with TTR ≥65%, dabigatran, and rivaroxaban (log‐rank: 59.7, P < 0.0001).
3.2. Ischemic stroke
There were 209 ischemic strokes in the study population, with an annual incidence of ischemic stroke of 4.03%/year. Figure 2A depicts the Kaplan‐Meier analysis of ischemic stroke among patients on warfarin and a NOAC, and Figure 2B depicts the analysis among patients on warfarin with TTR <65%, warfarin with TTR ≥65%, dabigatran, and rivaroxaban of different dosages (log‐rank: 31.9, P < 0.0001). The annual incidences of ischemic stroke were 4.53%/year and 2.82%/year among patients on warfarin and a NOAC. Of note, 150 strokes occurred in patients on warfarin with TTR <65% (4.95%/year), 17 strokes in patients with TTR ≥65% (2.57%/year), 28 in patients on rivaroxaban (3.74%/year), and 14 patients on dabigatran (1.89%/year) (Figure 2C). Of note, there was a difference in annual incidences of ischemic stroke in rivaroxaban of different dosages (6.74%/year at 15 mg daily and 1.93%/year at 20 mg daily). Comparatively, those on rivaroxaban at 20 mg daily had similar ischemic stroke risk compared to those on dabigatran.
Figure 2.
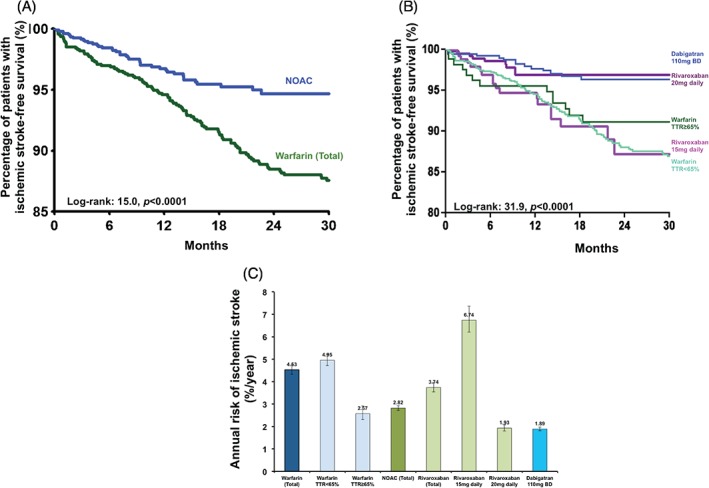
(A) Kaplan‐Meier estimates of ischemic stroke‐free survival in Chinese AF patients on warfarin and on a NOAC. (B) Kaplan‐Meier estimates of ischemic stroke‐free survival in Chinese AF patients on warfarin with TTR ≥65%, patients on warfarin with TTR <65%, patients on rivaroxaban 15 mg and 20 mg daily, and patients on dabigatran 110 mg twice daily. (C) The annual risk of ischemic stroke in Chinese AF patients on warfarin with TTR ≥65%, patients on warfarin with TTR <65%, patients on rivaroxaban 15 mg and 20 mg daily, and patients on dabigatran 110 mg twice daily.
Table 2 summarizes factors predictive of ischemic stroke among patients on warfarin with TTR ≥65%, dabigatran, and rivaroxaban together with the corresponding HRs and 95% CIs on univariate and multivariate analyses based on Cox proportional hazards models.
Table 2.
Associations between baseline factors and ischemic stroke
| No. of Ischemic Strokes | Univariate Analysis | Multivariate Analysis | |||
|---|---|---|---|---|---|
| HR (95% CI) | P Value | HR (95% CI) | P Value | ||
| Age | |||||
| <65 years | 4 | Reference | Reference | ||
| 65–74 years | 11 | 2.31 (0.74‐7.26) | 0.152 | 2.05 (0.65‐6.46) | 0.22 |
| ≥75 years | 40 | 4.87 (1.74‐13.62) | 0.0031 | 4.21 (1.49‐11.86) | 0.007 |
| Female | 26 | 1.12 (0.66‐1.91) | 0.665 | ||
| Hypertension | 50 | 3.37 (1.34‐8.44) | 0.011 | 2.56 (1.00‐0.65) | 0.051 |
| Diabetes mellitus | 18 | 1.32 (0.75‐2.32) | 0.336 | ||
| Heart failure | 15 | 1.26 (0.69‐2.27) | 0.239 | ||
| Coronary artery disease | 8 | 1.57 (0.74‐3.32) | 0.452 | ||
| Prior ischemic stroke | 18 | 1.19 (0.68‐2.09) | 0.544 | ||
| Antithrombotic therapy | |||||
| Warfarin TTR ≥65% | 17 | Reference | Reference | ||
| Rivaroxaban | 28 | 0.81 (0.41‐1.58) | 0.533 | 0.61 (0.31‐1.20) | 0.152 |
| Dabigatran | 14 | 0.43 (0.20‐0.93) | 0.0321 | 0.39 (0.18‐0.83) | 0.0151 |
Abbreviations: CI, confidence interval; HR, hazard ratio; TTR, time in therapeutic range.
Comparisons among patients on warfarin with TTR ≥65%, rivaroxaban, and dabigatran.
P < 0.05.
On univariate analysis, age ≥75 years (HR: 4.87, 95% CI: 1.74‐13.62, P = 0.003), and hypertension (HR: 3.37, 95% CI: 1.34‐8.44, P = 0.01) were associated with increasing risk of ischemic stroke. Compared with warfarin therapy with TTR ≥65%, the use of dabigatran was associated with lower ischemic stroke risk (HR: 0.43, 95% CI: 0.20‐0.93, P = 0.032). There was no statistically significant difference in ischemic stroke risk between rivaroxaban therapy and warfarin therapy with TTR ≥65% (HR: 0.81, 95% CI: 0.41‐1.58, P = 0.533).
On multivariate analysis, only age ≥75 years (HR: 4.21, 95% CI: 1.49‐11.86, P = 0.007) was associated with increasing risk of ischemic stroke, whereas and the use of dabigatran in comparison with warfarin with TTR ≥65% was associated with 61% reduction in ischemic stroke risk (HR: 0.39, 95% CI: 0.18‐0.83, P = 0.015). Further analyses comparing patients taking dabigatran and rivaroxaban found that the use of dabigatran was associated with lower ischemic stroke risk (HR: 0.52, 95% CI: 0.27‐0.99, P = 0.045).
3.3. Intracranial hemorrhage
There were 41 ICHs during the study period (0.76%/year); 34 occurred in patients on warfarin and the remaining 7 in patients on a NOAC. The annual incidences of ICH were 0.89%/year and 0.46%/year, respectively. Among patients on warfarin, a majority of ICHs (30 out of 34) occurred among those with TTR <65%, with an annual incidence of 0.95%/year, compared with only 0.58%/year for those with TTR ≥65%, 3 in patients on dabigatran, and 4 in patients on rivaroxaban. Figure 3A shows the Kaplan‐Meier analysis of ICH among patients on warfarin and a NOAC (log‐rank: 0.40, P = 0.53), and Figure 3B depicts the Kaplan‐Meier analysis of ICH among patients on warfarin with TTR <65%, warfarin with TTR ≥65%, dabigatran, and rivaroxaban (log‐rank: 3.892, P = 0.42). Of note, the annual incidences of ICH were 0.95%/year, 0.58%/year, 0.52%/year, and 0.39%/year among patients on warfarin with TTR <65%, warfarin with TTR ≥65%, rivaroxaban, and dabigatran, respectively (Figure 3C).
Figure 3.
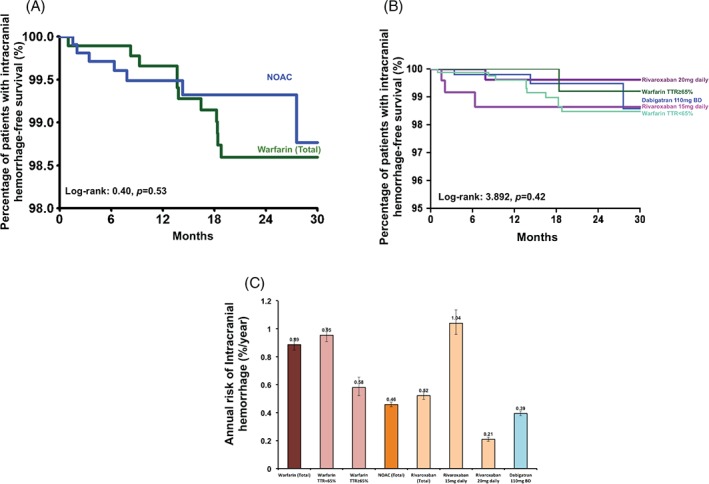
(A) Kaplan‐Meier estimates of intracranial hemorrhage‐free survival in Chinese AF patients on warfarin and on a NOAC. (B) Kaplan‐Meier estimates of intracranial hemorrhage‐free survival in Chinese AF patients on warfarin with TTR ≥65%, patients on warfarin with TTR <65%, patients on rivaroxaban 15 mg and 20 mg daily, and patients on dabigatran 110 mg twice daily. (C) The annual risk of intracranial hemorrhage in Chinese AF patients on warfarin with TTR ≥65%, patients on warfarin with TTR <65%, patients on rivaroxaban 15 mg and 20 mg daily, and patients on dabigatran 110 mg twice daily.
4. DISCUSSION
To our knowledge, this is the first study to compare the efficacy and safety of good control of warfarin therapy, dabigatran, and rivaroxaban in the Chinese patients with nonvalvular AF in a real‐world clinical setting. First, we reaffirmed the importance of good anticoagulation control of warfarin therapy for stroke prevention in AF, whereby patients on warfarin with TTR <65% had the highest ischemic stroke risk (5.24%/year) among all treatment groups despite comparable CHA2DS2‐VASc scores. Second, patients on dabigatran had a lower ischemic stroke risk compared with patients on warfarin with TTR ≥65% (57% relatively risk reduction) and rivaroxaban (48% relative risk reduction). There was no statistically significant difference in ischemic stroke risk between patients on warfarin with TTR <65% and patients on rivaroxaban 15 mg daily. In addition, there was no significant difference in ischemic stroke risk between those on rivaroxaban 20 mg daily and dabigatran 110 mg twice daily, which were regarded as standard dosages for these 2 NOACs. Although statistically nonsignificant due to the low event rates, patients on rivaroxaban 20 mg daily had the numerically lowest ICH risk (0.21%/year) compared with patients on dabigatran (0.39%/year), patients on warfarin with TTR ≥65% (0.58%/year), and patients on warfarin with TTR <65% (0.95%/year). Patients on rivaroxaban 15 mg daily had the highest ICH risk (1.04%/year).
The Chinese population is among the ethnicities with the highest ischemic stroke risk worldwide. For instance, the ischemic stroke risks in 2010 was 0.24%/year in China and 0.17%/year in Taiwan, in comparison to only 0.09%/year in the United Kingdom and 0.14%/year in the United States. It has been estimated that 1 out of 5 to 6 strokes in the Chinese population is related to AF. Although warfarin therapy has shown to be effective for stroke prevention in patients with AF for more than 20 years,3, 15 the difficulty of achieving good anticoagulation control among the Chinese population5, 16, 17 and the fear of ICH as a result of anticoagulation therapy5, 16, 17 remain as the 2 major stumbling blocks to stroke prevention in the Chinese AF population. As a result, anticoagulation utilization among Chinese AF patients has been as low as 2.7% to 19.8%.4 The importance of good‐quality anticoagulation, as usually defined by TTR ≥65%, among patients taking warfarin to prevent ischemic stroke,6 cannot be emphasized enough. Consistent with previous studies reporting the notoriously low TTR in Chinese and other Asian populations,6 in the present study involving 2099 Chinese AF patients in an all‐comer basis, only 16.3% of patients on warfarin achieved good anticoagulation (TTR ≥65%), which translated into a reduction of ischemic stroke risk by nearly 2%/year compared with patients on warfarin with TTR <65%. Equally important, patients with TTR <65% had a much higher ICH risk compared with those with TTR ≥65% (0.95%/year vs 0.58%/year).
In the 4 large phase III clinical trials, NOACs including dabigatran, rivaroxaban, apixaban, and edoxaban have shown at least to be noninferior to warfarin in preventing ischemic stroke; however, the corresponding ICH risk is only half of that of warfarin.18 Together with the predictable pharmacokinetics, NOACs can potentially circumvent many drawbacks of warfarin particularly in the Chinese AF population. Despite this, Chinese AF patients constitute only around 5% of the total study populations in major NOAC trials, disproportionate with the huge number of AF patients in the Chinese population.9, 10, 11 In the present study, 1 of the most striking findings is that compared with patients on warfarin with TTR ≥65%, Chinese AF patients on dabigatran (>85% on dabigatran 110 mg 2 times a day) had an additional 57% ischemic stroke risk reduction. This observation is in stark contrast to the RE‐LY trial, where among patients on dabigatran 150 mg twice daily, the relative risk reduction of ischemic stroke when compared with those on warfarin therapy was only 34%.9 More importantly, the observed difference in ischemic stroke risk between the present study and the RE‐LY trial may not be fully explained by the differences in demographic factors such as age (73.1 years vs 71.5 years), CHADS2 (congestive heart failure, hypertension, age ≥75 years, diabetes, previous stroke) score (2.4 vs 2.1), and TTR (77.5% vs 67%).9 However, our observation is somewhat consistent with the RE‐LY Asian substudy8 showing that dabigatran 110 mg twice daily was associated with a lower risk of ischemic stroke compared with warfarin (2.5%/year vs 3.06%/year, HR: 0.81). In non‐Asian populations participating in the RE‐LY trial, the difference in ischemic stroke risk between dabigatran and warfarin therapy was modest (1.37%/year vs 1.48%/year).8
In the present study, rivaroxaban also appears to be more potent for stroke prevention than that observed in the ROCKET‐AF trial.10 As in the latter trial,10 patients on rivaroxaban in the present study had a comparable ischemic stroke risk with those on warfarin with TTR ≥65% (3.74%/year vs 3.35%/year), although the mean TTR among patients on warfarin with good anticoagulation control in the present study was 77.5%, which was much higher than that in the ROCKET‐AF trial (58%). However, if only those on rivaroxaban 20 mg daily were selected for comparison, the annual incidence of ischemic stroke would be much lower than those on warfarin with TTR ≥65% (1.93%/year vs 3.35%/year). In the ROCKET‐AF East Asian substudy, East Asian AF patients randomized to rivaroxaban had a much lower ischemic stroke risk than those randomized to warfarin (2.6%/year vs 3.4%/year), whereas in non–East Asian counterparts, the risk did not differ much between those randomized to rivaroxaban and to warfarin (2.1%/year vs 2.4%/year).
Due to the lack of head‐to‐head comparison among NOACs in a randomized, control trial setting, international guidelines often consider all NOACs possessing equal or comparable efficacy and safety profile in their recommendations, and offer little guidance on the selection. In the present study, another important finding is that Chinese AF patients on dabigatran, as well as those on rivaroxaban 20 mg daily, had a significantly lower ischemic stroke risk as well as a numerically lower ICH risk compared to those on rivaroxban 15 mg daily, which accounted for 41% of patients taking rivaroxaban. Collectively, these data from the observational study, despite the fact that selection of anticoagulation were based on individual physician's decision, suggest that NOACs are preferentially indicated in the Chinese population.
4.1. Limitations
This study is limited by being based on single center and use of a hospitalized patient dataset. Because of the observational nature, the decision for and the selection of antithrombotic agents were not in a randomized fashion. Although a majority of patients on dabigatran was prescribed with the standard 110 mg twice daily standard dosage, 41% on rivaroxaban were on a reduced dosage, which might be based on other clinical consideration other than renal impairment (creatinine clearance 30–49 mL/min). Thereby, it is possible that residual confounding may be evident such that patients receiving different antithrombotic strategies, as well as patients on warfarin with different TTR were in some ways different from each other. Although patients receiving warfarin and patients receiving NOACs were broadly comparable in risk profile, as reflected by similar CHA2DS2‐VASc and HAS‐BLED scores, other factors not included in these scores had not been exhaustively sought. In addition, due to the observational nature, certain clinical parameters such as body mas index and actual blood pressure variation, and concomitant medications that may be relevant to the observed event rate were not available. Risk assessment was made at baseline, but the patients risk profile may change over time. Overall, low TTR in warfarin users was multifactorial but commonly observed in Asian/Chinese populations, even in the large‐scale randomized control trial setting and with vigorous attempts to maintain therapeutic INRs.
5. CONCLUSION
In Chinese AF patients, dabigatran therapy was associated with lower ischemic stroke (1.89%/year) and ICH rates (0.39%/year) compared to good‐control warfarin therapy. Rivaroxaban was comparable to warfarin therapy with TTR ≥65% in terms of ischemic stroke risk reduction and ICH, and those on rivaroxaban 20 mg daily had comparable efficacy and safety as those on dabigatran. Practically, the 2 NOACs in this study provide comparable alternatives to warfarin, particularly in populations with poor TTR (ie, <65%) where they were associated with higher safety and efficacy.
Conflicts of interest
Gregory Y. H. Lip, MD, the following: guideline membership/reviewing: European Society of Cardiology Guidelines on Atrial Fibrillation, 2010 and Focused Update, 2012; European Society of Cardiology Guidelines on Heart Failure, 2012; American College of Chest Physicians Antithrombotic Therapy Guidelines for Atrial Fibrillation, 2012; National Institute for Health and Care Excellence Guidelines on Atrial Fibrillation, 2006 and 2014; National Institute for Health and Care Excellence Quality Standards on Atrial Fibrillation 2015; European Society of Cardiology Cardio‐Oncology Task Force, 2015; European Society of Cardiology Working Group on Thrombosis position documents (2011–present). Chairman, Scientific Documents Committee, European Heart Rhythm Association. Dr. Lip is a reviewer for various guidelines and position statements from European Society of Cardiology, European Heart Rhythm Association, and National Institute for Health and Care Excellence. His participation in steering committees/trials includes steering committees for various phase II and III studies, and health economics and outcomes research. Dr. Lip is an investigator in various clinical trials in cardiovascular disease, including those on antithrombotic therapies in atrial fibrillation, acute coronary syndrome, and lipids; a consultant for Bayer/Janssen, Johnson & Johnson, Astellas, Merck, Sanofi, BMS/Pfizer, Biotronik, Medtronic, Portola, Boehringer Ingelheim, Microlife, and Daiichi‐Sankyo; and a speaker for Bayer, BMS/Pfizer, Medtronic, Boehringer Ingelheim, Microlife, Roche, and Daiichi‐Sankyo. The other authors have no conflicts of interest to disclose.
Li W‐H, Huang D, Chiang C‐E, Lau C‐P, Tse H‐F, Chan EW, Wong ICK, Lip GYH, Chan P‐H and Siu C‐W. Efficacy and safety of dabigatran, rivaroxaban, and warfarin for stroke prevention in Chinese patients with atrial fibrillation: the Hong Kong Atrial Fibrillation Project, Clin Cardiol, 2016. doi: 10.1002/clc.22649
Contributor Information
Pak‐Hei Chan, Email: phmchan@hku.hk.
Chung‐Wah Siu, Email: cwdsiu@hku.hk.
REFERENCES
- 1. Zhou Z, Hu D. An epidemiological study on the prevalence of atrial fibrillation in the Chinese population of mainland China. J Epidemiol. 2008;18:209–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Lau CP, Gbadebo TD, Connolly SJ, et al. Ethnic differences in atrial fibrillation identified using implanted cardiac devices. J Cardiovasc Electrophysiol. 2013;24:381–387. [DOI] [PubMed] [Google Scholar]
- 3. Camm AJ, Lip GY, De Caterina R, et al. 2012 focused update of the ESC Guidelines for the management of atrial fibrillation: an update of the 2010 ESC guidelines for the management of atrial fibrillation. Developed with the special contribution of the European Heart Rhythm Association. Eur Heart J. 2012;33:2719–2747. [DOI] [PubMed] [Google Scholar]
- 4. Siu CW, Lip GY, Lam KF, Tse HF. Risk of stroke and intracranial hemorrhage in 9727 Chinese with atrial fibrillation in Hong Kong. Heart Rhythm. 2014;11:1401–1408. [DOI] [PubMed] [Google Scholar]
- 5. Yang QD, Niu Q, Zhou YH, et al. Incidence of cerebral hemorrhage in the Changsha community. A prospective study from 1986 to 2000. Cerebrovasc Dis. 2004;17:303–313. [DOI] [PubMed] [Google Scholar]
- 6. Ho CW, Ho MH, Chan PH, et al. Ischemic stroke and intracranial hemorrhage with aspirin, dabigatran, and warfarin: impact of quality of anticoagulation control. Stroke. 2015;46:23–30. [DOI] [PubMed] [Google Scholar]
- 7. Lip GY, Wang KL, Chiang CE. Non‐vitamin K antagonist oral anticoagulants (NOACs) for stroke prevention in Asian patients with atrial fibrillation: time for a reappraisal. Int J Cardiol. 2015;180:246–254. [DOI] [PubMed] [Google Scholar]
- 8. Hori M, Connolly SJ, Zhu J, et al. Dabigatran versus warfarin: effects on ischemic and hemorrhagic strokes and bleeding in Asians and non‐Asians with atrial fibrillation. Stroke. 2013;44(7):1891–1896. [DOI] [PubMed] [Google Scholar]
- 9. Connolly SJ, Ezekowitz MD, Yusuf S, et al. Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med. 2009;361:1139–1151. [DOI] [PubMed] [Google Scholar]
- 10. Patel MR, Mahaffey KW, Garg J, et al. Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. N Engl J Med. 2011;365:883–891. [DOI] [PubMed] [Google Scholar]
- 11. Connolly SJ, Eikelboom J, Joyner C, et al. Apixaban in patients with atrial fibrillation. N Engl J Med. 2011;364:806–817. [DOI] [PubMed] [Google Scholar]
- 12. Siu CW, Tse HF. Net clinical benefit of warfarin therapy in elderly Chinese patients with atrial fibrillation. Circ Arrhythm Electrophysiol. 2014;7:300–306. [DOI] [PubMed] [Google Scholar]
- 13. Chan PH, Li WH, Hai JJ, Tse HF, Siu CW. Impact of antithrombotic therapy in atrial fibrillation on the presentation of coronary artery disease. PLoS One. 2015;10:e0131479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Chan KH, Ka‐Kit Leung G, Lau KK, et al. Predictive value of the HAS‐BLED score for the risk of recurrent intracranial hemorrhage after first spontaneous intracranial hemorrhage. World Neurosurg. 2014;82:e219–e223. [DOI] [PubMed] [Google Scholar]
- 15. Hart RG, Pearce LA, Aguilar MI. Meta‐analysis: antithrombotic therapy to prevent stroke in patients who have nonvalvular atrial fibrillation. Ann Intern Med. 2007;146:857–867. [DOI] [PubMed] [Google Scholar]
- 16. Zhang LF, Yang J, Hong Z, et al. Proportion of different subtypes of stroke in China. Stroke. 2003;34:2091–2096. [DOI] [PubMed] [Google Scholar]
- 17. Huang CY, Chan FL, Yu YL, Woo E, Chin D. Cerebrovascular disease in Hong Kong Chinese. Stroke. 1990;21:230–235. [DOI] [PubMed] [Google Scholar]
- 18. Arbit B, Hsu JC: Non‐vitamin K antagonist oral anticoagulant use in patients with atrial fibrillation and associated intracranial hemorrhage: a focused review. Clinical Cardiol. 2015;38:684–691. [DOI] [PMC free article] [PubMed] [Google Scholar]


