Figure 4.
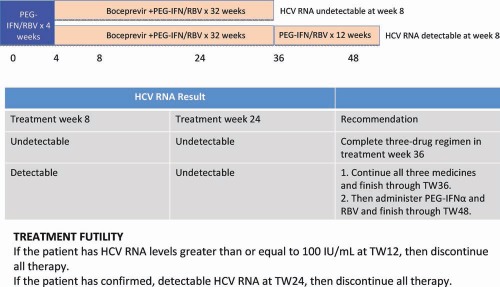
Boceprevir plus PEG‐IFN/RBV for previous partial responders and relapsers: the FDA‐approved regimen. Subjects should start treatment with PEG‐IFN and RBV for 4 weeks; after this, boceprevir (800 mg three times a day with food) is given. The duration of boceprevir use depends on the response to treatment at weeks 8 and 24. If HCV RNA is undetectable at weeks 8 and 24, patients should receive 32 weeks of triple therapy. If a patient is slow to respond and has detectable HCV RNA at week 8 but HCV RNA is undetectable at week 24, then triple therapy should be given for 32 weeks and should be followed by another 12 weeks of PEG‐IFN and RBV. TW, treatment week.
