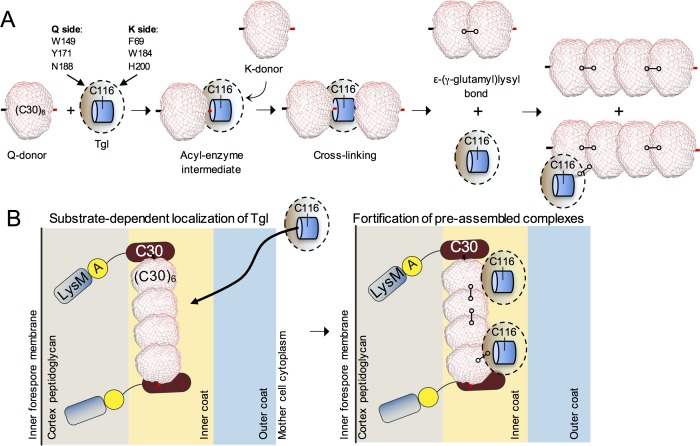
Fig 8. Control of Tgl localization and activity at the spore surface.
A: reactions of (C30)6 with Tgl. We propose that the (C30)6 hexamer acts both as a Q and K donor and becomes cross-linked by Tgl to form a dimer of hexamers. An intermediate step in this reaction (not represented for simplicity) appears to be the formation of cross-linked dimers within the (C30)6 hexamer, which may be a trimer of cross-linked dimers (see the Discussion for details). With time, this species becomes cross-linked into larger cross-linked oligomers, in what may represent de novo protein polymerization. Tgl itself is cross-linked into the forming structure. B: SafA is recruited early to the surface of the developing spore, prior to synthesis of Tgl. At the spore surface, SafA is found in at least two forms: SafAFL (full-length) and C30. SafAFL has a localization signal formed by a LysM domain and region A [30]. The protein is found in association with both the cortex peptidoglycan and in the inner coat [30] but only the first localization is depicted for simplicity. C30 interacts with SafAFL via the corresponding region in the latter. A likely interaction of SafAFL with itself is not represented. As C30 lacks localization signals, this interaction is required for the localization of C30 to the coat. SafAFL and C30 are key determinants in the recruitment of Tgl to the coat (left panel), placing the enzyme in close proximity to its substrates. Residues involved in substrate recognition, mainly in the Q side of the enzyme (light brown shading), are required for the localization of Tgl. In a second step, Tgl cross-links C30 (and presumably C30 to the corresponding region of SafAFL; not represented) to fortify pre-assembled complexes of SafAFL and C30. During the process, Tgl itself also becomes cross-linked, which may eventually limit its activity at the spore surface. The proteins are not drawn to scale.