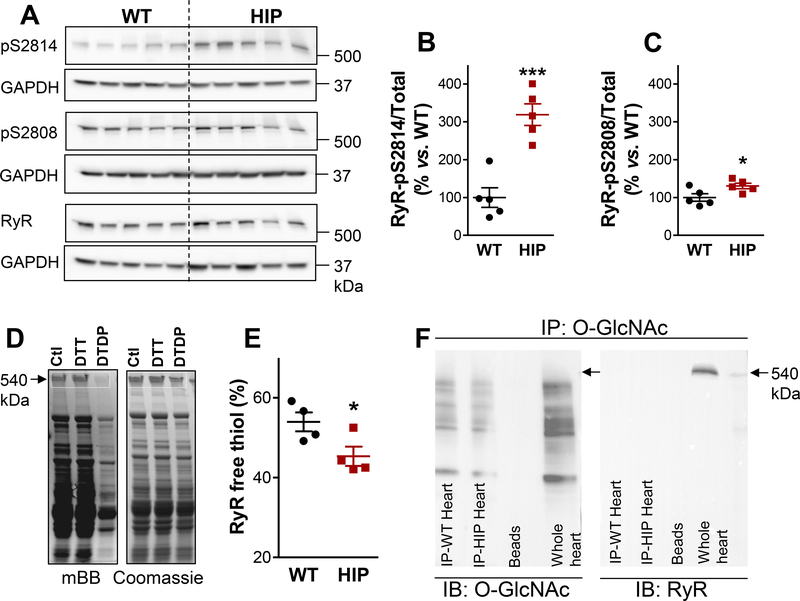
Figure 4. Ehanced RyR phosphorylation and oxidation in diabetic HIP rat hearts.
(A) Immunoblots on heart homogenates with antibodies that recognize RyR phosphorylated at the CaMKII (S2814) and PKA (S2808) sites and total RyR. GAPDH is the loading control. (B-C) Ratio of phosphorylated-to-total RyR for the S2814 (B) and S2808 (C) phosphorylation sites in HIP and WT hearts. N=5 hearts/group and immunoblots were repeated 4 times. (D) Measurement of free thiols contained in RyRs. Myocytes from each heart were divided in three groups, lysed and incubated under control conditions (Ctl), with the reducing agent dithiothreitol (DTT) or with the oxidizing agent 2,2’-dithiodipyridine (DTDP). Lysates were then incubated with mBB and loaded on two SDS-PAGE gels for measurements of mBB florescence and, respectively, protein content (by Coomassie Blue staining). (E) Percentage of RyR free thiols calculated from experiments as in panel D. Measurements were performed in duplicate on myocyte lysates from 4 WT and 4 HIP rats. (F) Measurement of RyR O-GlcNAcylation. O-GlcNAcylated proteins immunoprecipitated, separated by SDS-PAGE and probed with antibodies that recognize either OGlcNAc (left panel) or RyR (right panel). Whole heart homogenate was used as positive control for RyR (Whole heart column). Similar experiments were performed on homogenates from 3 WT and 3 HIP hearts.