Abstract
Objective
To determine if increasing predicted risk of cesarean was associated with longer labor length and increased morbidity among women undergoing induction with an unfavorable cervix.
Study Design
Using a publically available database, we evaluated whether a previously validated prediction model for cesarean delivery after induction was associated with labor length, maternal morbidity (third-/fourth-degree lacerations, endometritis, blood transfusion, wound infection, venous thromboembolism, hysterectomy, intensive care unit admission, and death), and neonatal morbidity (blood transfusion, encephalopathy, intraventricular hemorrhage, severe respiratory distress syndrome, necrotizing enterocolitis, and sepsis). Full-term (≥37 weeks) singleton gestations with intact membranes and an unfavorable cervix (Bishop score ≤6 and dilation ≤2 cm) undergoing induction of labor were included.
Results
A total of 8,466 women met the inclusion criteria. Each category increase in cesarean probability (<20, 20–39.9, 40–59.9, ≥60%) was associated with an increase in labor length (9.6, 10.8, 11.7, and 11.9 hours, respectively; p < 0.001). With increasing predicted probability of cesarean there, was also a significant increase in maternal morbidity with each category (2.6, 4.7, 5.1, 6.1%; p = 0.001) and increase in neonatal morbidity (0.9, 1.5, 2, 2.2%; p = 0.002).
Conclusion
Using a validated prediction model for cesarean delivery among women induced with an unfavorable cervix, increasing predicted probability of cesarean is associated with longer labor length and increased maternal and neonatal morbidity.
Keywords: calculator, cesarean section, induction of labor, labor length, maternal morbidity, neonatal morbidity, unfavorable cervix
Induction of labor, which will subsequently be referred to as induction, is one of the most common obstetrical procedures, affecting up to 1 million pregnant women in the United States per year.1 Although approximately one-third of inductions will end in a cesarean delivery,2–5 we have been limited in our ability to predict which women undergoing an induction will ultimately have a cesarean. Recently, however, a validated prediction model for cesarean delivery after labor induction was developed based on five variables known at the start of induction (height, body mass index (BMI), parity, gestational age at induction, and starting Bishop score).6
Identifying women at a high risk of cesarean delivery is important as cesarean deliveries are associated with increased maternal morbidity such as obstetric hemorrhage, surgical site infection, and venous thromboembolism.7 Furthermore, prolonged labor, regardless of the mode of delivery, is associated with maternal morbidity.8,9 Cesarean delivery and prolonged labor have also both been associated with higher neonatal morbidity, such as neonatal respiratory distress and admission to the neonatal intensive care unit (NICU).8–10 It would therefore be beneficial for both patients and providers to be able to predict which women are not only at a high risk of cesarean delivery but also at a risk of prolonged labor and maternal and neonatal morbidity.
Therefore, using a validated cesarean prediction calculator, we aimed to determine if increasing risk of cesarean was associated with longer labor length and increased maternal and neonatal morbidity among women undergoing an induction with an unfavorable cervix.
Methods
A calculator for predicting the risk of cesarean among women undergoing an induction of labor with an unfavorable cervix was derived and internally validated using data from the University of Pennsylvania.6 In bivariate analysis, the following variables had a p < 0.20 and were considered in the development of the prediction model: maternal height, weight gain during pregnancy, BMI at delivery, race, parity, gestational age at induction, indication for induction, pre-existing diabetes, chronic hypertension, cervical dilation at start of induction, and the modified Bishop scores at the start of induction. In the multivariable modeling, nulliparity, gestational age at induction ≥40 weeks, BMI category at delivery, the modified Bishop score at induction, and height category were the five variables that remained significantly associated with cesarean. The calculator was then externally validated using the Consortium of Safe Labor, which is a publicly available National Institutes of Health database that includes labor and delivery information collected from the electronic medical record for more than 200,000 deliveries from 19 hospitals across the United States between 2002 and 2008. The final area under the receiver operating characteristic curve was 0.73 (95% confidence interval: 0.72–0.74). This calculator is publicly available (http://www.uphs.upenn.edu/obgyn/labor-induction-calculator/).
For this study, we used this calculator to evaluate labor and delivery outcomes within the Consortium of Safe Labor population. The same inclusion and exclusion criteria that were used in the parent study to derive and validate the calculator were used for this analysis.6 Briefly, we included full-term (≥37 weeks) women carrying a singleton gestation with intact membranes and an unfavorable cervix (Bishop score ≤6 and dilation ≤2 cm) who were undergoing an induction. Women with a prior cesarean section were excluded.
Women were grouped into categories based on the predicted risk of cesarean at the start of the labor induction (<20, 20–39.9, 40–59.9, ≤60%). Primary outcomes evaluated are as follows (1): length of labor (defined as time from start of induction to delivery) (2); composite maternal morbidity (defined as one or more of the following: third- or fourth-degree lacerations, endometritis, blood transfusion, wound infection, venous thromboembolism, hysterectomy, intensive care unit admission, and death) (3); composite neonatal morbidity (defined as one or more of the following: blood transfusion, encephalopathy, intraventricular hemorrhage, severe respiratory distress syndrome, necrotizing enterocolitis, and sepsis).
Secondary outcomes evaluated were maternal length of stay (LOS), maternal prolonged LOS > 3 days after delivery, maternal prolonged LOS > 5 days after delivery, and neonatal NICU admission > 48 hours.
Descriptive statistics for continuous variables are presented as mean and standard deviation or median and interquartile range, where appropriate. Categorical variables are presented as frequencies and percentages. Bivariate analyses were performed using Fisher’s exact test for categorical variables and Kruskal–Wallis’ tests for continuous variables. Cox regression models were used to estimate adjusted hazard ratios for time to delivery, censored for cesarean. Logistic regression was used to estimate odds ratios for all other outcomes (maternal morbidity, mortality, prolonged LOS). All models were adjusted for maternal age, race, study site, and insurance status. Statistical analyses were performed with STATA version 15 (Stata Corp., College Station, TX).
Results
There were 8,466 women who met the inclusion criteria for this study. Baseline maternal characteristics for our cohort are shown in ►Table 1. The overall cesarean rate was 26.4%.
Table 1.
Maternal characteristics
| Maternal characteristics | n (%) |
|---|---|
| Age | 28 (6) |
| Race | |
| Caucasian | 3,789 (44.8) |
| African-American | 1,969 (23.3) |
| Hispanic | 1,826 (21.6) |
| Other | 882 (10.4) |
| Insurance | |
| Private | 4,131 (48.8) |
| Public | 2,027 (23.9) |
| Other | 149 (1.8) |
| Unknown | 2,159 (25.5) |
| BMI category | |
| < 25 | 1,071 (12.6) |
| 25–29.9 | 3,230 (38.1) |
| 30–34.9 | 2,306 (27.2) |
| 35–39.9 | 1,089 (12.9) |
| 40+ | 770 (9.1) |
| Maternal height category | |
| < 62 | 1,867 (22) |
| 62–63.9 | 1,457 (17.2) |
| 64–65.9 | 2,285 (27) |
| 66+ | 2,857 (33.7) |
| Pregestational diabetes | 159 (1.9) |
| Gestational diabetes | 540 (6.4) |
| Chronic hypertension | 186 (2.2) |
| Gestational hypertension/preeclampsia | 813 (9.8) |
| Nulliparous | 5,639 (66.6) |
| Cervical dilation at first exam | 1 (0.5–2) |
| Modified Bishop score, median (IQR) | 3 (2–5) |
| GA at delivery, median (IQR) | 39 (38–40) |
| Indication for induction | |
| Postdate | 965 (11.4) |
| Maternal | 909 (10.7) |
| Fetal | 939 (11.1) |
| Elective | 1,431 (16.9) |
| Unknown | 4,213 (49.8) |
Abbreviations: BMI, body mass index; GA, gestational age; IQR, inter-quartile range.
Using the validated calculator for cesarean delivery prediction, 45% of women had a predicted probability of 20%, 36% had a probability of 20 to 39.9%, 15% had a probability of 40 to 59.9%, and <5% had a probability ≥60% (►Fig. 1). Labor duration data were available for 6,886 (81.3%) of the 8,466. Each category increase in cesarean probability (<20, 20–39.9, 40–59.9, ≥60%) was associated with an increase in labor length (9.6, 10.8, 11.7, and 11.9 hours, respectively; p < 0.001). When censored for cesarean and adjusting for confounders including age, race, insurance type, and site, women with a higher predicted risk of cesarean continued to have a significantly longer time to delivery (Kaplan–Meier curve; ►Fig. 2).
Fig. 1.
Study population by the probability of cesarean using validated cesarean prediction calculator. This pie chart depicts how our study population was grouped by the predicted probability of cesarean using the validated calculator.
Fig. 2.
Labor length based on the predicted probability of cesarean. The Kaplan–Meier curve demonstrates time from induction start to delivery by cesarean probability group.
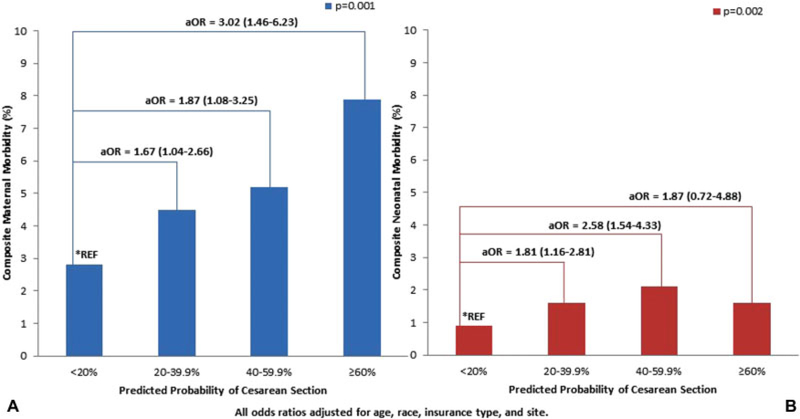
Maternal morbidity data were available for 2,780 (32.8%) of the 8,466; neonatal morbidity data were available for all 8,466 women (►Table 2). The overall rate of our maternal morbidity composite was 4.1%. As seen in ►Fig. 3A, there was a significant increase in the composite maternal morbidity outcome with increasing predicted probability of cesarean (p = 0.001). When adjusted for confounders and compared with women with a predicted cesarean probability of <20%, women with a predicted probability of 20 to 39.9% had a 65% increased risk, women with a predicted probability of 40 to 59.9% had an 85% increased risk, and women with a predicted probability of ≥60% had a 300% increased risk of maternal morbidity. When limiting our analysis to women with a vaginal delivery, the association of increased maternal morbidity with increased probability of cesarean remained (predicted probability: <20, 20–39.9, 40–59.9, ≥60; maternal morbidity: 2.6, 5, 5.1, 5.3, respectively; p = 0.01)
Table 2.
| Risk of cesarean section by calculator | |||||
|---|---|---|---|---|---|
| <20% | 20–39.9% | 40–59.9% | ≥60% | p-Value | |
| Composite maternal morbidity (n = 2,780) | 31(2.8) | 48(4.5) | 25(5.2) | 11(7.9) | 0.001 |
| Third-/fourth-degree lacerations | 13 (1.2) | 27 (2.6) | 9 (1.9) | 2 (1.4) | 0.11 |
| Endometritis | 4 (0.4) | 6 (0.6) | 8 (1.7) | 5 (3.6) | 0.001 |
| Blood transfusion | 12 (1.1) | 15 (1.4) | 7 (1.4) | 6 (4.3) | 0.05 |
| Wound infection | 0 | 1 (0.1) | 2 (0.4) | 0 | 0.14 |
| Venous thromboembolism | 0 | 0 | 2 (0.4) | 0 | 0.05 |
| Hysterectomy | 0 | 0 | 0 | 0 | − |
| ICU admission | 2 (0.2) | 3 (0.3) | 2 (0.4) | 1 (0.7) | 0.40 |
| Maternal death | 0 | 0 | 0 | 0 | − |
| Composite neonatal morbidity (n = 8,466) | 36(0.9) | 49(1.6) | 28(2.1) | 5(1.6) | 0.002 |
| Blood transfusion | 2 (0.05) | 1 (0.03) | 0 | 0 | 0.99 |
| Encephalopathy | 0 | 1 (0.03) | 0 | 0 | 0.55 |
| Intraventricular hemorrhage | 2 (0.05) | 3 (0.10 | 2 (0.15) | 0 | 0.54 |
| Severe respiratory distress syndrome | 14 (0.4) | 18 (0.6) | 7 (0.5) | 2 (0.6) | 0.46 |
| Necrotizing enterocolitis | 0 | 0 | 0 | 0 | − |
| Sepsis | 21 (0.5) | 32 (1) | 18 (1.4) | 4 (1.3) | 0.01 |
Abbreviation: ICU, intensive care unit.
Adjusted for age, race, insurance type, and site.
Individual components of the morbidity are included in the composite only once.
Fig. 3.
Predicted probability of cesarean section and maternal and neonatal morbidity. (A) Predicted probability of cesarean section and composite maternal morbidity. (B) Predicted probability of cesarean section and composite neonatal morbidity.
The overall rate of our neonatal morbidity composite was 1.4%. As seen in ►Fig. 3B, there was a significant increase in the composite neonatal morbidity outcome with increasing predicted probability of cesarean (p = 0.002). When limited to only women with vaginal delivery, this increased risk was not significant (p = 0.21). When adjusted for confounders and compared with women with a predicted cesarean probability of <20%, women with a predicted cesarean probability of 20 to 39.9% had an 80% increased risk and those with a predicted probability of 40 to 59.9% had a 250% increased risk of neonatal morbidity. There was no significant difference in neonatal risk with ≤60% predicted probability of cesarean when compared with the lowest risk group.
Secondary outcomes are seen in ►Table 3. Increasing predicted probability of cesarean was also associated with a longer maternal LOS, an increased odds of prolonged maternal LOS > 3 and > 5 days, and increased odds of NICU admission > 48 hours. When limited to women who had a vaginal delivery, there remained an association with increased predicted probability of cesarean and maternal LOS (p < 0.001) and prolonged maternal LOS > 3 days (p < 0.001).
Table 3.
Secondary outcomes
| PP of cesarean section | Maternal PP LOS >3 days (n = 7,645), N (%) | Adjusted odds ratioa (CI) | Maternal PP LOS >5 days (n = 7,645), N (%) | Adjusted odds ratioa (CI) | NICU > 48 N (%) | Adjusted odds ratioa (CI) |
|---|---|---|---|---|---|---|
| <20% | 418/3,420 (12.2) | Ref | 43/3,420 (1.3) | Ref | 86 (2.3) | Ref |
| 20–40% | 851/2,713 (31.4) | 3.51 (3.07–4.02) | 78/2,713 (2.9) | 2.46 (1.67–3.61) | 89 (2.9) | 1.34 (0.98–1.82) |
| 40–60% | 612/1,206 (50.7) | 7.90 (6.73–9.28) | 62/1,206 (5.1) | 4.24 (2.82–6.38) | 59 (4.6) | 2.05 (1.44–2.91) |
| 60–80% | 196/306 (64) | 13.73 (10.5–17.89) | 37/306 (12.1) | 10.90 (6.75–17.58) | 16 (5.1) | 2.17 (1.24–3.81) |
| p-Value | <0.001 | <0.001 | <0.001 |
Abbreviations: CI, confidence interval; LOS, length of stay; NICU, neonatal intensive care unit; PP, predicted probability.
Adjusted for age, race, insurance type, and site.
Discussion
When using a well-validated cesarean prediction calculator among women undergoing an induction with an unfavorable cervix, we demonstrated that increasing risk of cesarean was associated with longer labor length and increased maternal and neonatal morbidity. Specifically, women with predicted probability of cesarean of ≥60% had a threefold higher odds of maternal morbidity compared with women with a predicted probability of <20%. This increased morbidity risk holds true even for women who ultimately have a vaginal delivery, suggesting there is something intrinsic to their morbidity risk that is not solely explained by the mode of delivery.
The cesarean risk prediction calculator used in this study incorporates five variables known at the start of induction (height, BMI, parity, gestational age at induction, and starting Bishop score). Although these individual variables have been associated with morbidity in prior studies,11,12 data that have quantified the specific risk of maternal and neonatal morbidity given individual patient characteristics are limited. Our study has significant clinical implications for both providers and patients. First, these results can augment counseling of women regarding the risks and expectations surrounding an induction. Women and providers can benefit from the knowledge of associations between the calculator result and length of labor. This knowledge may assist women in mental and emotional preparation for the induction and delivery process. Furthermore, realistic expectations may improve the patient’s ultimate satisfaction with the induction experience. In a shared approach, knowledge about expected length of labor can also be critical as providers make decisions in labor, such as those regarding labor interventions and timing of cesarean deliveries. An understanding of the calculator’s associations with maternal and neonatal morbidity is also necessary for thorough counseling of a patient at the start of induction. This information could allow providers to prepare for such morbidities, for example, providers might use this information to guide a type and cross for blood products or alert higher level pediatric providers to increased neonatal risk. It is critical that the calculator is not used in isolation but rather used in the context of a woman’s clinical information including indication for induction, maternal/fetal well-being, and progress in labor.
This study uses a large, contemporary population, with deliveries from diverse geographic locations and practice types, which increases generalizability of our results. Our study is limited by the intrinsic limitations of the calculator; these data only apply to women with term, singleton gestations undergoing induction of labor with intact membranes and unfavorable cervices. Furthermore, the Consortium for Safe Labor database is a publically available, deidentified database. Indication for induction and complete maternal morbidity data was only available on a fraction of the population. However, it is important to note that the missing data are due to variation in collection procedures by participating site, not individually missing patients. In addition, the prevalence of neonatal outcomes was low and therefore underpowered to see differences in subgroup analysis including when stratifying by the mode of delivery.
Future studies should evaluate whether implementation of the calculator into clinical care, through augmentation of clinical decision-making, can reduce maternal and neonatal morbidity without impacting cesarean delivery rate. For example, for women with a high predicted risk of cesarean, providers may makethe decision for cesarean sooner than they would have without calculator knowledge, which would lead to the prevention of lengthy and prolonged inductions associated with morbidity. Lastly, it is important to evaluate whether a woman’s knowledge of the calculator results improves her satisfaction with the labor and delivery process.
Acknowledgments
The data included in this study were obtained from the Consortium on Safe Labor, supported by the Intramural Research Program of the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD), National Institutes of Health, through Contract No. HHSN267200603425C. Institutions involved in the Consortium include, in alphabetical order, the following: Baystate Medical Center, Springfield, MA; Cedars-Sinai Medical Center Burns and Allen Research Center, Los Angeles, CA; Christiana Care Health System, Newark, DE; Georgetown University Hospital, MedStar Health, Washington, DC; Indiana University Clarian Health, Indianapolis, IN; Intermountain Healthcare and the University of Utah, Salt Lake City, Utah; Maimonides Medical Center, Brooklyn, NY; MetroHealth Medical Center, Cleveland, OH.; Summa Health System, Akron City Hospital, Akron, OH; The EMMES Corporation, Rockville MD (Data Coordinating Center); University of Illinois at Chicago, Chicago, IL; University of Miami, Miami, FL; and University of Texas Health Science Center at Houston, Houston, Texas. The named authors alone are responsible for the views expressed in this manuscript, which does not necessarily represent the decisions or the stated policy of the NICHD. Wewould like to thank the principal investigators who conducted the original Consortium of Safe Labor study, as well as the NICHD for funding the study and NICHD DASH for providing us with the data.
Funding
This study was funded in part by a career development award in Women’s Reproductive Health Research: K12-HD001265-16 [LL].
Footnotes
Conflict of Interest
None declared.
References
- 1.Center for Disease Control and Prevention. Recent declines in induction of labor Available at: http://www.cdc.gov/nchs/data/databriefs/db155.htm. Accessed March 1, 2016
- 2.Rouse DJ, Weiner SJ, Bloom SL, et al. ; Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) Maternal-Fetal Medicine Units Network (MFMU). Failed labor induction: toward an objective diagnosis. Obstet Gynecol 2011;117(2 Pt 1):267–272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vahratian A, Zhang J, Troendle JF, Sciscione AC, Hoffman MK. Labor progression and risk of cesarean delivery in electively induced nulliparas. Obstet Gynecol 2005;105(04):698–704 [DOI] [PubMed] [Google Scholar]
- 4.Grobman WA. Predictors of induction success. Semin Perinatol 2012;36(05):344–347 [DOI] [PubMed] [Google Scholar]
- 5.Tolcher MC, Holbert MR, Weaver AL, et al. Predicting cesarean delivery after induction of labor among nulliparous women at term. Obstet Gynecol 2015;126(05):1059–1068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Levine LD, Downes KL, Parry S, Elovitz MA, Sammel MD, Srinivas SK. A validated calculator to estimate risk of cesarean after an induction of labor with an unfavorable cervix. Am J Obstet Gynecol 2018;218 (02):254.e1–254.e7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Spong CY, Berghella V, Wenstrom KD, Mercer BM, Saade GR. Preventing the first cesarean delivery: summary of a joint Eunice Kennedy Shriver National Institute of Child Health and Human Development, Society for Maternal-Fetal Medicine, and American College of Obstetricians and Gynecologists Workshop. Obstet Gynecol 2012;120(05):1181–1193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cheng YW, Shaffer BL, Bryant AS, Caughey AB. Length of the first stage of labor and associated perinatal outcomes in nulliparous women. Obstet Gynecol 2010;116(05):1127–1135 [DOI] [PubMed] [Google Scholar]
- 9.Maghoma J, Buchmann EJ. Maternal and fetal risks associated with prolonged latent phase of labour. J Obstet Gynaecol 2002;22 (01):16–19 [DOI] [PubMed] [Google Scholar]
- 10.Rouse DJ, McCullough C, Wren AL, Owen J, Hauth JC. Active-phase labor arrest: a randomized trial of chorioamnion management. Obstet Gynecol 1994;83(06):937–940 [DOI] [PubMed] [Google Scholar]
- 11.Lisonkova S, Muraca GM, Potts J, et al. Association between prepregnancy body mass index and severe maternal morbidity. JAMA 2017;318(18):1777–1786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Grobman WA, Bailit JL, Rice MM, et al. ; Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) Maternal-Fetal Medicine Units (MFMU) Network. Frequency of and factors associated with severe maternal morbidity. Obstet Gynecol 2014;123(04):804–81024785608 [Google Scholar]