Fig. 6.
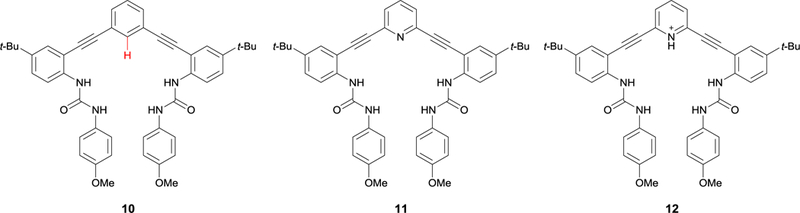
The three anion receptors that brought our lab into its current generation of aryl CH hydrogen bond studies. Tresca et al. compared the binding affinities of the phenyl- (10), pyridine- (11), and pyridinium-core (12) receptors to realize the potential of the supporting aryl CH HB in our scaffolds.
