Abstract
The recent microcephaly outbreak in Brazil has been associated with Zika virus (ZIKV) infection. The current understanding of damage caused by ZIKV infection is still unclear, since it has been implicated in other neurodegenerative and developmental complications. Here, the differential proteome analysis of human mesenchymal stem cells (hMSC) infected with a Brazilian strain of ZIKV was identified by shotgun proteomics (MudPIT). Our results indicate that ZIKV induces a potential reprogramming of the metabolic machinery in nucleotide metabolism, changes in the energy production via glycolysis and other metabolic pathways, and potentially inhibits autophagy, neurogenesis, and immune response by down-regulation of signaling pathways. In addition, proteins previously described in several brain pathologies, such as Alzheimer’s disease, autism spectrum disorder, amyotrophic lateral sclerosis, and Parkinson’s disease, among others, were found with altered expression due to ZIKV infection in hMSC. This potential link between ZIKV and several neuropathologies beyond microcephaly is being described here for the first time and can be used to guide specific follow-up studies concerning these specific diseases and ZIKV infection.
Keywords: Zika virus, Brain diseases, Human Mesenchymal Stem Cells, Proteome, Microcephaly
Introduction
Zika virus (ZIKV) belongs to the family Flaviviridae, genus Flavivirus, and is a single-stranded RNA virus that is currently getting major attention from the scientific community and health agencies around the world. The pathological potential of ZIKV was revealed in 2015 due to a microcephaly outbreak occurring in Brazil that was linked to its infection [1, 2]. Since then, ZIKV has been implicated into other neurological diseases worldwide.
Shotgun proteomics using Multidimensional Protein Identification Technology (MudPIT) [3] is a reliable way to characterize biological changes focusing on effects of environmental or physiological induction of gene expression. Differential proteomics has been applied to characterize molecular alterations promoted by various stimuli, such as drugs or pathological states. The rational use of proteomics is also widely applied to identify potential biomarkers of diseases and can reveal the molecular basis of host-pathogen interactions [3–6]. Unfortunately, despite the state of global health emergency on ZIKV disease declared by World Health Organization (WHO) and the intense attention of the scientific community since the Brazilian microcephaly outbreak, the potential of proteomics to understand ZIKV infections has been largely ignored. To our knowledge, only one paper has been published on global proteomics of ZIKV infection presenting interesting findings using an elegant and singular model of human neurospheres [7]. This lack of proteomics papers on ZIKV is surprising because this approach would be very helpful to identify genes, proteins, signaling pathways and molecular processes linked to ZIKV’s deleterious effects on different cells, animal models or tissues.
In this report, human mesenchymal stem cells (hMSC) were infected with a Brazilian strain of ZIKV isolated during the Brazilian outbreak. hMSC are multipotent cells that have the capacity to differentiate into mature cell types including neurons [8], which are cells strongly affected by ZIKV infection [9]. Recently, it was shown that ZIKV has presented tropism to hMSC [10]. Microcephaly and the neurological disturbances observed in congenital ZIKV infections might be explained at least partially by an abnormal differentiation of infected stem/progenitor cells [10]. This postulation reinforces the urgent need of molecular information of stem cells infected by ZIKV. Our results present a substantial dataset containing hundreds of proteins from hMSC with expression altered due to ZIKV infection. Among these, we could molecularly assign several proteins with previously reported ZIKV clinical consequences like microcephaly and other well-known brain disorders, revealing dozens of new molecular targets for future studies.
Materials and methods
Ethics Statement
The study was approved by the institutional research ethics committee of Hospital de Clínicas de Porto Alegre (Federal University of Rio Grande do Sul) under protocol # 2018-0059.
ZIKV
The ZIKV strain 17 SM used in this work was isolated in 2016, in São Paulo city, Brazil, from a 33 years old woman who reported fever with duration of 2 days, followed by headache and retro-orbital pain (days 2–3), and a maculopapular rash accompanied by an intense itching and diffuse arthralgia (on day 4). The 17 SM ZIKV strain was propagated in African green monkey kidney (VERO) cells (ATCC CRL-1586) and tittered expressed as plaque-forming units (PFU). Viral stocks were stored at −80 °C until use.
Human mesenchymal stem cells (hMSC) cultures
After obtaining written informed consent, tissues were collected from three healthy donors at Hospital de Clínicas de Porto Alegre, Brazil and cells were isolated [11]. The cell suspension was plated and maintained in culture in low glucose-Dulbecco’s modified Eagle’s medium (Gibco, Grand Island, NY, USA) containing 1% penicilin-streptomycin (Gibco, Grand Island, NY, USA) and 20% fetal calf serum (Gibco, Grand Island, NY, USA) at 37 °C in a humidified 5% CO2 atmosphere. The isolated cells developed to visible systematic colonies of adherent fibroblast-like cells at about 2–5 days after initial plating [11]. These cells were characterized by their adhesiveness, fibroblastoid shape and ability to in vitro differentiate into adipocytes, osteocytes and chondrocytes [12, 13]. Immunophenotypic identification for CD105, CD73, CD90, CD44, CD45, CD34, CD11b, CD19 and HLA-DR were performed (BD Stemflow hMSC Analysis Kit, BD Biosciences). Related isotype antibodies were used as control. The analysis was performed using FACSCanto II (BD Biosciences) and FlowJo software (FlowJo LLC).
ZIKV infection of hMSC
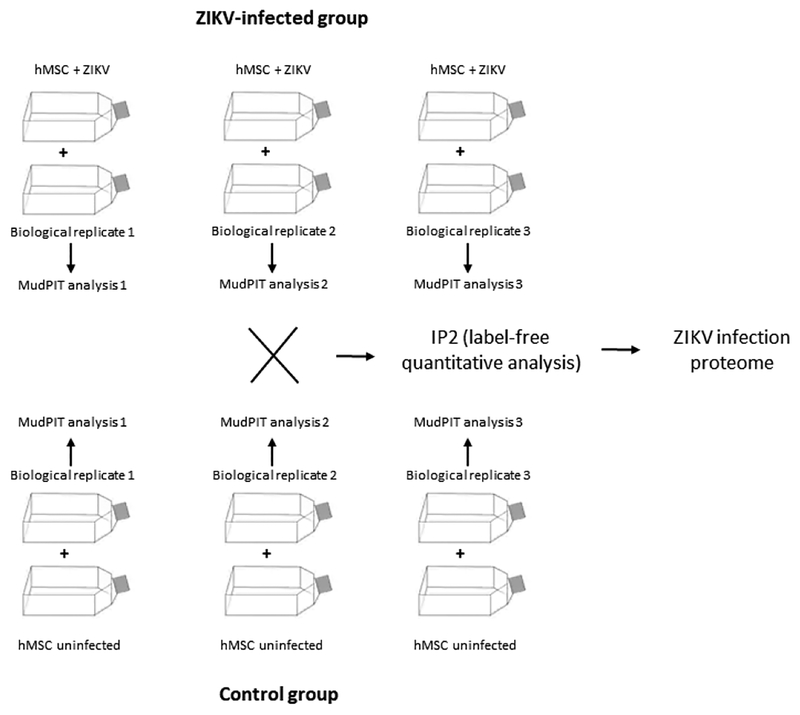
hMSC were infected at a multiplicity of infection (MOI) of 10, with an adsorption period of 2 h at 37 °C in T75 culture flasks. Subsequently, the inoculum was replaced by fresh DMEM supplemented with penicillin (20 units/mL), streptomycin (20 mg/mL), and 10% (v/v) heat-inactivated fetal bovine serum (all from Gibco, Gaithersburg, MD), and maintained at 37 °C in a 5% CO2 atmosphere. Two days post-infection, the medium was removed and PBS buffer was added. Then, the cells were scraped off, pelleted (5 min, 2,000 rpm) and washed once again in PBS. Mock-infected cells were used as controls. All experiments were performed in 3 independent biological replicates for both analytical groups (Fig.1).
Figure 1. Experimental design used to identify the differential proteome of ZIKV infection on hMSC.
Protein extraction and digestion for mass spectrometry
hMSC samples, uninfected (control) and ZIKV infected cells, were suspended in 100 uL of extraction buffer (8 M urea, 50 mM ammonium bicarbonate, 50 mM HEPES, pH 7.5), containing protease inhibitor cocktail plus EDTA (Thermo Scientific, Rockford, IL). The samples were then sonicated 10 min in ultrasonic bath, following 3 cycles (20 sec each) using a tip sonicator (intervals of 1 min on ice after each sonication round). After sonication, samples were centrifuged (14,000 rpm, 10 min, 4 °C) and the supernatants were recovered. Protein concentration was determined using BCA assay kit (Thermo Scientific, Rockford, IL). Approximately 100 μg of samples were suspended in digestion buffer (8 M urea, 100 mM tris-HCl pH 8.5) and treated as previously described [4]. The proteins were digested with 2 μg of trypsin (Promega, Madison, WI) by incubation at 37 °C during 16 h. Proteolysis was stopped by adding formic acid to a final concentration of 5%. Samples were centrifuged at 14,000 rpm for 20 min, and the supernatant was collected and stored at −80 °C.
MudPIT and mass spectrometry analyses
Briefly, the protein digest was analyzed using a modified 7-step MudPIT separation as previously described [14], ranging from 0 to 100% buffer C. Peptides eluting from the microcapillary column were electrosprayed directly into an LTQ Orbitrap XL mass spectrometer (Thermo Fisher Scientific, San Jose, CA). The search was made using ProLuCID algorithm [15] against human and Zika reviewed proteins list from UniProt (downloaded in August 22nd, 2017). ProLuCID results were assembled and filtered using the DTASelect program [16] resulting in a data set with a false discovery rate of 1% for protein. The MudPIT and mass spectrometry analysis are fully described in Supplementary File 1.
Spectrum raw files were extracted into MS1 and MS2 files using RawConverter [17]. To estimate peptide probabilities and FDRs accurately, we used a target/decoy database containing the reversed sequences of all the proteins appended to the target database. Tandem mass spectra were matched to sequences using the ProLuCID algorithm with 50 ppm peptide mass tolerance for precursor ions and 400 ppm for fragment ions. The search space included all fully and half-tryptic peptide candidates that fell within the mass tolerance window with no miscleavage constraints. Carbamidomethylation (+57.02146 Da) of cysteine was considered as a static modification. The validity of peptide/spectrum matches (PSMs) was assessed in DTASelect using two SEQUEST-defined parameters, the cross-correlation score (XCorr), and normalized difference in cross-correlation scores (DeltaCN). The search results were grouped by charge state (+1, +2, +3, and greater than +3) and tryptic status (fully tryptic, half-tryptic, and nontryptic), resulting in 12 distinct subgroups. In each of these subgroups, the distribution of Xcorr, DeltaCN, and DeltaMass values for targeted and decoy database PSMs was obtained; then the targeted and decoy subsets were separated by quadratic discriminant analysis. We performed label-free quantitative analysis using Census through IP2 (Integrated Proteomics Applications, Inc., http://www.integratedproteomics.com) [18]. First, the elemental compositions and corresponding isotopic distributions for each peptide were calculated, and this information was then used to determine the appropriate m/z range from which to extract ion intensities, which included all isotopes with greater than 5% of the calculated isotope cluster base peak abundance. MS1 files were used to generate chromatograms from the m/z range surrounding precursor peptides.
We grouped biological replicates of each sample (Fig.1) to determine the protein level. Census used protein identification results from DTASelect2 and generated a reconstructed MS1 based chromatogram for each identified peptide. When peptides are not identified in all the relevant samples, Census searched missing peptides using accurate precursor mass, retention time, and charge states and retrieve them and build their chromatograms. We generated peptide ratios by randomly choosing pairs of peptide intensities from the 3 biological replicates for the two different samples. The Grubbs test (p value < 0.01) was then applied to remove outlier peptides. To increase accuracy for finding peptide precursors, we calculated Pearson product-moment correlation coefficient comparing theoretical and experimental isotope distributions to minimize false peak detection. When peaks are not detected, we calculated background noise to assign small values to peptides.
For all further bioinformatics analysis, all proteins with negative fold change and presenting p<0.05 (ratio p-value in Supplementary Table 2) or that were uniquely identified in control condition were considered down-regulated and all proteins with positive fold change and presenting p<0.05 or that were uniquely identified in infected hMSC were considered up-regulated. The p-value (p<0.05) was calculated based on the normal distribution of the observed protein ratios by using Census software.
Pathway mapping and functional annotation of the differential proteome
The FASTA files with identified protein sequences (418 up and 867 down) were given, separately, as input to Blast2GO tool (version 5.1.12) (http://www.blast2go.com) for BLAST annotation [19]. As part of the Blast2GO analysis, proteins were assigned with an EC (Enzyme Code) number and mapped to the relevant Kyoto Encyclopedia of Genes and Genomes (KEGG) pathways.
The Panther database (http://www.pantherdb.org) was used to categorize the identified proteins in different levels: biological process, molecular function and cellular component [20], according to Gene Ontology (GO) terms (http://www.geneontology.org), an explicit evolutionary framework to infer annotation of proteins from a broad set of genomes from experimental annotations in a semi-automated manner.
Correlation and assignment of identified proteins to brain diseases and interactome analysis
To investigate which differentially expressed proteins identified were related to previously described clinical phenotypes and other brain pathologies, comparisons were made between identified proteins and those related to the pathogenesis of human brain diseases. Accordingly, the MalaCards database of human diseases [21] was used, selecting seven clinical phenotypes previously associated to ZIKV infection and 23 others brain diseases (Supplementary Table 1) notoriously known for comparison [22]. The search was made manually for each protein identified against all proteins correlated to those selected diseases.
Further, a protein-protein network analysis of the proteins assigned specifically to those brain diseases was also performed submitting the corresponding protein IDs to the STRING software (version 10.5) (http://stringdb.org/) [23]. For each protein-protein association stored in STRING, a score is provided. These scores (i.e., the ‘edge weights’ in each network) represent confidence scores, and are scaled between zero and one. Proteins were represented with nodes and the interactions with continuous lines showing direct interactions (physical), all being numerically quantified (node score). The node scores indicate the estimated likelihood that a given interaction is biologically meaningful, specific and reproducible, given the supporting evidence. For each interaction, the supporting evidence is divided into one or more ‘evidence channels’, depending on the origin and type of the evidence. All the edges were supported by at least a reference from the literature or from canonical information stored in the STRING database. Cytoscape software (version 3.6.1) [24] was applied to visualize the protein-protein interaction relationship network. Centiscape 2.2 application in Cytoscape was used to calculate the degree related with the predicted regulatory relevance of each node [25].
Results
Differential proteome overview of hMSC infected with ZIKV
A total of 2,518 proteins were identified in the hMSC proteome when infected with ZIKV (Supplementary Table 2). Among these proteins, 145 were considered differentially expressed (p<0.05), with 27 up-regulated and 118 down-regulated, compared to control cells. Moreover, 295 and 635 proteins were uniquely identified in infected hMSC and in control cells, respectively.
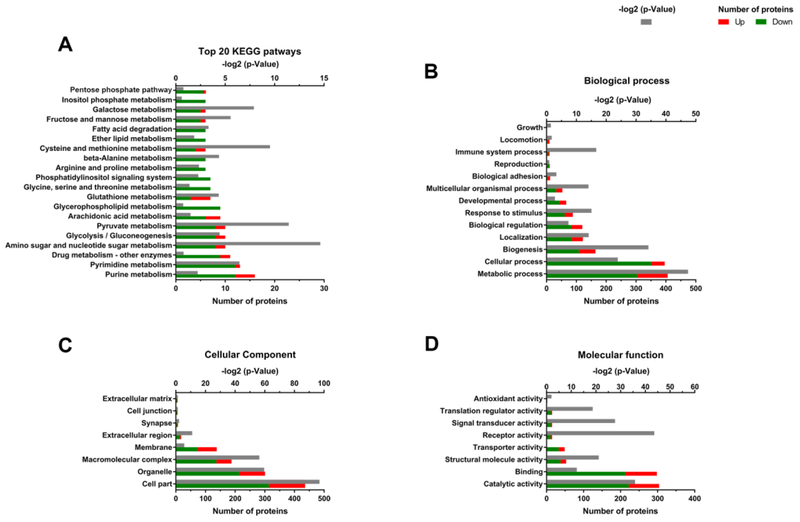
The top 20 most affected pathways, in number of proteins, are presented in Fig. 2A, most of them relate to energy metabolism and nucleotide pathways. Proteins related to metabolism of purines, amino acids (glycine, serine and threonine), lipids (phosphatidylinositol) as well as signaling pathways, such as phosphoinositide 3 kinase (PI3K) were down-regulated upon infection. Proteins were assigned according to Gene Ontology (GO) levels (Cellular Component, Molecular Function, Biological Process) based on their expression (Fig. 2B, C and D). Proteins involved in cellular and metabolic processes and related to binding and catalytic activities were mostly down-regulated. For proteins categorized as cellular components (Fig. 2C) the majority of the proteins were assigned to organelles, macromolecular complexes or membranes.
Figure 2. Functional characterization of differential proteome of hMSC infected with ZIKV.
A) top 20 most impacted KEGG pathways, in number of proteins. Gene ontology assignment of proteins in different levels: B) biological process, C) molecular function, and D) cellular component. For these analyses, all proteins with negative fold change and presenting p<0.05 or that were uniquely identified in control condition were considered down-regulated and all proteins with positive fold change and presenting p<0.05 or that were uniquely identified in infected hMSC were considered up-regulated (Supplementary Table 1).
Brain diseases protein correlation
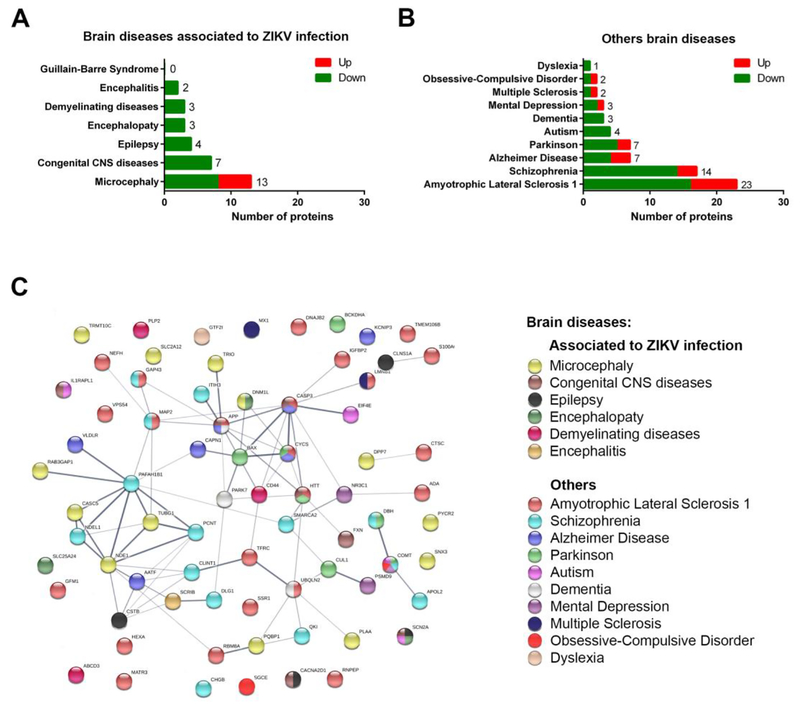
All proteins identified in the proteome were manually checked in the MalaCards database, to correlate protein molecular annotation to ZIKV pathological consequences and to other well-known brain diseases. The highest number of proteins assigned to pathological phenotypes already associated to ZIKV, were found related to microcephaly with 13 protein references identified, followed by congenital central nervous system diseases and epilepsy (Fig. 3A; Supplementary Table 3). Proteins related to other brain diseases not yet related to ZIKV infection were found. These proteins in order of numbers were related to amyotrophic lateral sclerosis (ALS), with 23 protein references, followed by schizophrenia, Alzheimer, Parkinson diseases and autism (Fig. 3B). All proteins associated with brain diseases were used in a protein-protein interaction network analysis (Fig. 3C). The interactome showed a close association between proteins related to microcephaly and schizophrenia, ALS, Alzheimer and Parkinson disease, and dementia.
Figure 3. ZIKV Differentially Expressed Proteins (DEPs) associated with human brain diseases.
Number of proteins assigned to brain diseases A) previously associated to ZIKV infection or B) assigned to other brain diseases. C) protein-protein interaction network of proteins showed in A and B. Proteins were represented by nodes and the interactions with continuous lines, where thicker lines represent stronger interaction. Proteins presented here were considered statistically (p>0.05) differentially regulated (up- or down-regulated or unique).
We also attributed degrees to proteins in the interactome (Supplementary Table 3). The biological meaning of the degree is the potential regulatory relevance of each node. Proteins with higher degree interact with multiple proteins, thus suggesting a central regulatory role, being considered as a protein hub, such as PAFAH1B1, NDE1, CASP3 and APP. For instance, CASP3, corresponding to caspase 3 protein involved in apoptosis, is associated with congenital CNS disease, Alzheimer and ALS. This protein interacts with 10 other proteins, most already described in ALS (Fig. 3C). In the same way, PAFAH1B1, corresponding to platelet-activating factor acetylhydrolase, associated with schizophrenia, interacts with proteins previously associated with microcephaly and schizophrenia.
Discussion
While different aspects of ZIKV pathology are undergoing scientific study, the molecular process of infection remains poorly characterized. A global proteomic view of hMSC ZIKV infection points to metabolic pathways being most impacted and describes new molecular pathways and potential mechanisms to be investigated. By considering the entire proteomic dataset, 101 different pathways were found affected by ZIKV infection. In other cells, it has been also shown that some pathways are disturbed by ZIKV infection [26–28], suggesting a potential cell reprograming towards nucleotide synthesis, energy metabolism, signaling pathways, among others [7, 26], similarly to the pattern found in our results.
Interestingly, proteins from signaling pathways including PI3K and mTOR (Supplementary Fig. 1 and Supplementary Fig. 2) were found inhibited by ZIKV infection, which was previously shown to culminate in alterations in autophagy, neurogenesis, and immune response [29, 30]. Brain malformations, such as microcephaly, are associated with alterations in PI3K/Akt and mTOR pathways [29], leading to increase viral proliferation due to inhibition of PI3K, a classical pathway associated to cellular proliferation [30]. Moreover, PI3K/Akt and mTOR are closely associated to several other pathways that maintain brain homeostasis. In addition, both might involve cellular dysfunction, inflammatory response, impairment in redox status with consequent nitrosative/oxidative stress and metabolic alterations. In this sense, PI3K is a crucial signal to triggering inflammatory response, through NFκB transcriptional activity in neural cells [31–33]. These pathways might coordinate eukaryotic cell growth and metabolism, including nutrients and growth factors [34]. Thus, these signaling pathways could be the key in cellular events linked to microcephaly and the progression of neurological disorders potentially associated with ZIKV.
To ensure optimal environments for their replication and spread, viruses have evolved to alter many host cell pathways [35], especially those related with nucleotide metabolism. It results in increased nucleotide biosynthesis, thus providing substrates for synthesis of purine and pyrimidine for viral replication, [36, 37]. In addition, most viruses evaluated by metabolomics induce alterations in cellular metabolism, including fatty acid synthesis, among others. These modifications of carbon source utilization by infected cells can increase available energy for virus replication and virion production, creating viral replication niches while increase infected cell survival [35], corroborating with KEGG results. In the same way, metabolic alterations induced by ZIKV infection culminate in the overexpression or accumulation of some metabolites, including lipids [29]. As observed in the phosphatidylinositol signaling system (Supplementary Fig. 3), proteins from this pathway were found altered with potential accumulation of two lipids, PI (1-phosphatidyl-D-myo-inositol) and PIP2 (phosphatidylinositol-4,5-bisphosphate), already described as possible biomarkers of ZIKV-infected patients [29]. Therefore, ZIKV infection has induced alterations in different signaling pathways, which have culminated with the overexpression of some metabolites, including above-reported lipids.
Taken together, our data and the above-described data, these effects can be noticed not only in a wider view (metabolic pathways), but also in specific gene/protein identifications. For example, we have identified in hMSC 88 proteins corresponding to 69 genes previously identified with altered expression due to ZIKV infection in four different cell types [26]. Comparing hMSC results with a neurosphere dataset [7], there are 219 shared protein identifications. Nevertheless, our proteome analysis presents a set of novel proteins, molecular processes and pathways affected by ZIKV infection in hMSC. Also, the majority of the studies until now have identified host-virus interactions by identifying genes affected through mRNA transcriptional profiling. However, proteomic data is closer to the physiological processes to better understand the biological phenomena and mechanisms [4, 38]. Furthermore, several studies note the poor correlation between mRNA and protein levels [39–41] suggesting that to understand the molecular mechanisms of the host-virus interaction requires measurements at the proteome level.
Since ZIKV effects were shown to be mainly promoted in the brain and have presented clear brain clinical phenotypes, like microcephaly [1, 22, 29], our results point to a potential link to other unexpected brain diseases according to protein neuropathological annotation. The association of diseases with metabolic routes, genes and signaling pathways are a central theme of present-day research scrutiny and it is very helpful for more meaningful conclusions and selection of molecular targets for additional studies, including biomarkers, and potential therapeutic strategies [21, 42]. After searching for correlations between the proteins identified in our study against 30 different brain disorders, 74 different proteins were found implicated in 16 neuropathologies (six out of seven phenotypes already associated to ZIKV infection, as expected, and 10 out of 23 to other brain diseases). Furthermore, although with different clinical symptomatology, the brain disorders associated to ZIKV infection described here share some cellular and molecular mechanisms; thus, the proteomic analyses might improve the understanding of pathomechanisms of brain diseases. A protein interaction network was built only with these proteins to improve the selection of potential candidates for future deep surveys, since they are already known in the context of brain pathologies. Moreover, the identification of specific biomarkers/targets not only can increase the understanding of the molecular basis of ZIKV pathology, but also may link to potential new clinical consequences, unknown yet, since most of the individuals infected in the outbreak are under surveillance. Proteins like CASP3, APP, NDE1, CYCS and PAFAH1B1 are central nodes interacting with multiple proteins being potential targets to be further analyzed. In line with this, NDE1 protein, which presented higher degree in our network, is related to cognitive disability, a crucial event in Alzheimer’s disease, as well as APP and CASP3 proteins, which are associated with neuronal death in the same condition [43]. In addition, MAP2 stabilizes dendritic shape during neuron development/survival and CYCS regulates mitochondrial functionality, including apoptosis [44]. In the interactome, MAP2 is a node linking two major hubs: CASP3 and PAFAH1B1. CASP3 is the predominant caspase involved in the cleavage of amyloid-beta 4A precursor protein, which is associated with neuronal death in Alzheimer’s disease [45]. In the same way, PAFAH1B1, which regulates the amount of platelet activating factor (PAF) in the brain, a protein involved in neuronal migration, is essential for normal brain development and function [46]. Thus, we proposed the association between specific proteins altered in our experimental model and brain disorders, independent of microcephaly. These proteins are also strongly linked to cellular components, such as synaptic and membrane functions (Fig. 2C) [47]. Moreover, PI3K/Akt regulates membrane trafficking of several neural proteins, including glutamate transporters that are involved in the pathogenesis of several brain diseases [48].
In addition, a new observation of monitored subjects is important to check any new potential clinical phenotype, as it was detected in our molecular data. It is surprising the increased number of proteins found implicated in neuropathologies not yet related to ZIKV infection. Anyway, the cloudy panel of ZIKV infection and pathology can lead to speculation based on molecular data on potential brain disorders usually happening later in life. For instance, ZIKV alters the DNA methylome of multiple cells at genes that have been implicated in the pathogenesis of a number of neuropathologies, like schizophrenia [49]. Accordingly, this disease was the second most prominent in number of correlated-proteins in our analysis. It can certainly lead to preventive assumption of all these potential phenotypes and improve diagnosis and therapeutics of individuals that are still suffering with undergoing neurological impairment due to ZIKV.
The understanding of fundamental mechanisms of ZIKV pathogenesis in hMSC could help future strategies to prevent ZIKV infections and its consequent negative effects [10, 50]. Our report is adding a substantial amount of new of protein information about the impact of ZIKV on the molecular pathways of hMSC that are disrupted and are related to brain diseases that will be very helpful in increasing our understanding of ZIKV infection and associated-diseases. This information can be used in preventive clinical surveys for new pathological phenotypes helping in treatment and diminishing the associated damage of ZIKV neurological disorders.
Supplementary Material
Acknowledgements
The authors would like to thank Dr. E. Durigon, ICB/USP, for supplying the ZIKV strain. This work was suported by the Brazilian funding agencies Coordenação de Aperfeiçoamento Pessoal de Nível Superior (CAPES), FAPERGS, Edital MCTIC/FNDCT-CNPq/ MEC-CAPES/ MS-Decit / No 14/2016, project 440763/2016-9. The study was also supported by NIH grants NIH/NIHGM P41 GM103533-22 and NIH/NIMH 5 R01 MH067880-14 (to JRY). PMR is a 1A CNPq research fellow. APMV and TFT acknowledges postdoctoral fellowship support by CNPq/HCPA.
Footnotes
Compliance with Ethical Standards
The authors declare no conflict of interest. The study was approved by the institutional research ethics committee of Hospital de Clínicas de Porto Alegre (Federal University of Rio Grande do Sul) under protocol # 2018-0059.
References
- 1.França GV, Schuler-Faccini L, Oliveira WK, Henriques CM, Carmo EH, Pedi VD, et al. (2016). Congenital Zika virus syndrome in Brazil: a case series of the first 1501 livebirths with complete investigation. Lancet 388(10047):891–897. doi: 10.1016/S0140-6736(16)30902-3 [DOI] [PubMed] [Google Scholar]
- 2.Schuler-Faccini L, Ribeiro EM, Feitosa IM, et al. (2016) Possible association between Zika virus infection and microcephaly - Brazil, 2015. MMWR Morb Mortal Wkly Rep 65:59–62. doi: 10.15585/mmwr.mm6503e2 [DOI] [PubMed] [Google Scholar]
- 3.McDonald WH, Yates JR 3rd (2002) Shotgun proteomics and biomarker discovery. Dis Markers 18(2):99–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Beys-da-Silva WO, Santi L, Berger M, Calzolari D, Passos DO, Guimarães JA, et al. (2014) Secretome of the biocontrol agent Metarhizium anisopliae induced by the cuticle of the cotton pest Dysdercus peruvianus reveals new insights into infection. J Proteome Res 13:2282–2296. doi: 10.1021/pr401204y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Santi L, Beys-da-Silva WO, Berger M, Calzolari D, Guimarães JA, Moresco JJ, Yates JR (2014) Proteomic profile of Cryptococcus neoformans biofilm reveals changes in metabolic processes. J Proteome R 13:1545–1559. doi: 10.1021/pr401075f [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Berger M, Santi L, Beys-da-Silva WO, Oliveira FMS, Caliari MV, Yates JR, et al. (2015) Mechanisms of acute kidney injury induced by experimental Lonomia obliqua envenomation. Arch Toxicol 89:459–483. doi: 10.1007/s00204-014-1264-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Garcez PP, Nascimento JM, Vasconcelos JM, Costa RM, Delvecchio R, Trindade P, et al. (2017) Zika virus disrupts molecular fingerprinting of human neurospheres. Sci Rep 7:40780. doi: 10.1038/srep40780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zomer HD, Vidane AS, Gonçalves NN, Ambrosio CE (2015) Mesenchymal and induced pluripotent stem cells: general insights and clinical perspectives. Stem Cells Cloning 8:125–134. doi: 10.2147/SCCAA.S88036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Olmo IG, Carvalho TG, Costa VV, Alves-Silva J, Ferrari CZ, Izidoro-Toledo TC, et al. (2017) Zika virus promotes neuronal cell death in a non-cell autonomous manner by triggering the release of neurotoxic factors. Front Immunol 8:1016. doi: 10.3389/fimmu.2017.01016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.El Costa H, Gouilly J, Mansoy J-M, Chen Q, Levy C, Cartron G, et al. (2016) ZIKA virus reveals broad tissue and cell tropism during the first trimester of pregnancy. Sci Rep 6:35296. doi: 10.1038/srep35296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zuk PA, Zhu M, Ashjian P, et al. (2002) Human adipose tissue is a source of multipotent stem cells. Mol Biol Cell 13:4279–4295. doi: 10.1091/mbc.e02-02-0105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Meirelles LDS, Nardi NB (2003) Murine marrow-derived mesenchymal stem cell: isolation, in vitro expansion, and characterization. Br J Haematol 123(4):702–711. [DOI] [PubMed] [Google Scholar]
- 13.Terraciano P, Garcez T, Ayres L, Durli I, Baggio M, Kuhl CP, et al. (2014) Cell therapy for chemically induced ovarian failure in mice. Stem Cells Int 2014:720753. doi: 10.1155/2014/720753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Washburn MP, Wolters D, Yates JR 3rd (2001) Large-scale analysis of the yeast proteome by multidimensional protein identification technology. Nat Biotechnol 19 (3):242–247. doi: 10.1038/85686 [DOI] [PubMed] [Google Scholar]
- 15.Xu T, Venable JD, Park SK, Cociorva D, Lu B, Liao L, et al. (2006) ProLuCID, a fast and sensitive tandem mass spectra-based protein identification program. Mol Cell Proteomics 5:S174. [Google Scholar]
- 16.Tabb DL, McDonald WH, Yates JR 3rd (2002) DTASelect and Contrast: tools for assembling and comparing protein identifications from shotgun proteomics. J Proteome Res 1:21–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.He L, Diedrich JK, Chu YY, Yates JR (2015) Extracting accurate precursor information for tandem mass spectra by RawConverter. Anal Chem 87 (22):11361–11367. doi: 10.1021/acs.analchem.5b02721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Park SK, Venable JD, Xu T, Yates JR 3rd (2008) A quantitative analysis software tool for mass spectrometry-based proteomics. Nat Methods 5(4):319–322. doi: 10.1038/nmeth.1195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Götz S, García-Gómez JM, Terol J, Williams TD, Nagaraj SH, Nueda MJ, et al. (2008) High-throughput functional annotation and data mining with the Blast2GO suite. Nucleic Acids Res 36(10):3420–3435. doi: 10.1093/nar/gkn176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gaudet P, Livstone MS, Lewis SE, Thomas PD (2011) Phylogenetic-based propagation of functional annotations within the Gene Ontology consortium. Brief Bioinform 12(5):449–462. doi: 10.1093/bib/bbr042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rappaport N, Nativ N, Stelzer G, Twik M, Guan-Golan Y, Stein TI, et al. (2013) MalaCards: an integrated compendium for diseases and their annotation. Database 2013:018. doi: 10.1093/database/bat018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rolfe AJ, Bosco DB, Wang J, Nowakowski RS, Fan J, Ren Y (2016) Bioinformatic analysis reveals the expression of unique transcriptomic signatures in Zika virus infected human neural stem cells. Cell Biosci 6:42. doi: 10.1186/s13578-016-0110-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Szklarczyk D, Morris JH, Cook H, Kuhn M, Wyder S, Simonovic M, et al. (2017) The STRING database in 2017: quality-controlled protein–protein association networks, made broadly accessible. Nucleic Acids Res 45:D362–D368. doi: 10.1093/nar/gkw937 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shannon P, Markiel A, Ozier O, Baliga NS, Wang JT, Ramage D, et al. (2003) Cytoscape: a software environment for integrated models of biomolecular interaction networks. Genome Res 13(11):2498–2504. 10.1101/gr.1239303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Scardoni G, Tosadori G, Faizan M, Spoto F, Fabbri F, Laudanna C (2014) Biological network analysis with CentiScaPe: centralities and experimental dataset integration. F1000Research 3, 139 10.12688/f1000research.4477.2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tiwari SK, Dang J, Qin Y, Lichinchi G, Bansal V, Rana TM (2017) Zika virus infection reprograms global transcription of host cells to allow sustained infection. Emerg Microbes Infect 6(4):e24. doi: 10.1038/emi.2017.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Brahma R, Gurumayum S, Naorem LD, Muthaiyan M, Gopal J, Venkatesan A (2018) Identification of hub genes and pathways in Zika virus infection using rna-seq data: a network-based computational approach. Viral Immunol 31(4):321–332. doi: 10.1089/vim.2017.0116. [DOI] [PubMed] [Google Scholar]
- 28.Caires-Júnior LC, Goulart E, Melo US, Araujo BHS, Alvizi L, Soares-Schanoski A, et al. (2018) Discordant congenital Zika syndrome twins show differential in vitro viral susceptibility of neural progenitor cells. Nat Commun 9(1):475. doi: 10.1038/s41467-017-02790-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Melo CFOR, Delafiori J, Oliveira DN, Guerreiro TM, Esteves CZ, et al. (2017) Serum metabolic alterations upon Zika infection. Front Microbiol 8:2373. doi: 10.3389/fmicb.2017.01954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang L, Yang L, Fikrig E, Wang P (2017) An essential role of PI3K in the control of West Nile virus infection. Sci Rep 7:3724. doi: 10.1038/s41598-017-03912-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nakajima S, Kitamura M (2013) Bidirectional regulation of NF-kappaB by reactive oxygen species: a role of unfolded protein response. Free Radic Biol Med 65:162–174. doi: 10.1016/j.freeradbiomed.2013.06.020. [DOI] [PubMed] [Google Scholar]
- 32.Kaur U, Banerjee P, Bir A, Sinha M, Biswas A, Chakrabarti S (2015) Reactive oxygen species, redox signaling and neuroinflammation in Alzheimer’s disease: the NF-kappaB connection. Curr Top Med Chem 15:446–457. doi: 10.2174/1568026615666150114160543 [DOI] [PubMed] [Google Scholar]
- 33.Gorina R, Font-Nieves M, Marquez-Kisinousky L, Santalucia T, Planas AM (2011) Astrocyte TLR4 activation induces a proinflammatory environment through the interplay between MyD88-dependent NFkappaB signaling, MAPK, and Jak1/Stat1 pathways. Glia 59:242–255. 10.1002/glia.21094 [DOI] [PubMed] [Google Scholar]
- 34.Saxton RA, Sabatini DM (2017) mTOR signaling in growth, metabolism, and disease. Cell 168(6):960–976. doi: 10.1016/j.cell.2017.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sanchez EL, Lagunoff M (2015) Viral activation of cellular metabolism. Virology 479–480:609–618. doi: 10.1016/j.virol.2015.02.038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Qimron U, Tabor S, Richardson CC (2010) New details about bacteriophage T7-host interactions. Microbe 5(3):117–122. [Google Scholar]
- 37.Enav H, Mandel-Gutfreund Y, Béjà O (2014) Comparative metagenomic analyses reveal viral-induced shifts of host metabolism towards nucleotide biosynthesis Microbiome 2:9. doi: 10.1186/2049-2618-2-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schrimpf SP, Weiss M, Reiter L, Ahrens CH, Jovanovic M, Malmström J, et al. (2009) Comparative functional analysis of the Caenorhabditis elegans and Drosophila melanogaster proteomes. PLoS Biol 7(3):e48. doi: 10.1371/journal.pbio.1000048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schwanhausser B, Busse D, Li N, Dittmar G, Schuchhardt J, Wolf J, et al. (2011) Global quantification of mammalian gene expression control. Nature 473(7347):337–342. doi: 10.1038/nature11848. [DOI] [PubMed] [Google Scholar]
- 40.de Sousa Abreu R, Penalva LO, Marcotte EM, Vogel C (2009) Global signatures of protein and mRNA expression levels. Mol Biosyst 5:1512–1526. doi: 10.1039/b908315d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Maier T, Guell M, Serrano L (2009). Correlation of mRNA and protein in complex biological samples. FEBS Lett 583:3966–3973. doi: 10.1016/j.febslet.2009.10.036. [DOI] [PubMed] [Google Scholar]
- 42.Rappaport N, Twik M, Plaschkes I, Nudel R, Stein TI, Levitt J, et al. (2017) MalaCards: an amalgamated human disease compendium with diverse clinical and genetic annotation and structured search. Nucleic Acids Res 45:D877–D887. doi: 10.1093/nar/gkw1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sadleir KR, Vassar R (2012) Cdk5 protein inhibition and Aβ42 increase bace1 protein level in primary neurons by a posttranscriptional mechanism implications of cdk5 as a therapeutic target for Alzheimer disease. J Biol Chem 287(10):7224–35. doi: 10.1074/jbc.M111.333914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sontag JM, Nunbhakdi-Craig V, White CL, Halpain S, Sontag E (2012) The protein phosphatase pp2a/bα binds to the microtubule-associated proteins Tau and MAP2 at a motif also recognized by the kinase Fyn: implications for tauopathies. J Biol Chem 287(18):14984–14993. doi: 10.1074/jbc.M111.338681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.D´Amelio M, Cavallucci V, Middei S, Marchetti C, Pacioni S, Ferri A, et al. (2011) Caspase-3 triggers early synaptic dysfunction in a mouse model of Alzheimer’s disease. Nature Neurosci 14:69–76. doi: 10.1038/nn.2709. [DOI] [PubMed] [Google Scholar]
- 46.Liu JS (2011) Molecular genetics of neuronal migration disorders. Curr Neurol Neurosci Rep 11(2):171–8. doi: 10.1007/s11910-010-0176-5. [DOI] [PubMed] [Google Scholar]
- 47.McNamara CR, Degterev A (2011) Small-molecule inhibitors of the PI3K signaling network. Future Med Chem 3(5):549–65. doi: 10.4155/fmc.11.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zelenaia O, Schlag BD, Gochenauer GE, Ganel R, Song W, Beesley JS, et al. (2000) Epidermal growth factor receptor agonists increase expression of glutamate transporter GLT-1 in astrocytes through pathways dependent on phosphatidylinositol 3-kinase and transcription factor NF-kappaB. Mol Pharmacol 57:667–678. 10.1124/mol.57.4.667 [DOI] [PubMed] [Google Scholar]
- 49.Janssens S, Schotsaert M, Karnik R, Balasubramaniam V, Dejosez M, Meissner A, et al. (2018) Zika virus alters DNA methylation of neural genes in an organoid model of the developing human brain. mSystems. 3(1):e00219–17. doi: 10.1128/mSystems.00219-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Schuler-Faccini L, Roehe P, Zimmer ER, Quincozes-Santos A, de Assis AM, Lima EOC, et al. (2018) ZIKA virus and neuroscience: the need for a translational collaboration. Mol Neurobiol. 55(2):1551–1555. doi: 10.1007/s12035-017-0429-2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.