Abstract
Background
Gestational diabetes is a type of diabetes that occurs during pregnancy. Women with gestational diabetes are more likely to experience adverse health outcomes such as pre‐eclampsia or polyhydramnios (excess amniotic fluid). Their babies are also more likely to have health complications such as macrosomia (birthweight > 4000 g) and being large‐for‐gestational age (birthweight above the 90th percentile for gestational age). Current clinical guidelines support elective birth, at or near term in women with gestational diabetes to minimise perinatal complications, especially those related to macrosomia.
This review replaces a review previously published in 2001 that included "diabetic pregnant women", which has now been split into two reviews. This current review focuses on pregnant women with gestational diabetes and a sister review focuses on women with pre‐existing diabetes (Type 1 or Type 2).
Objectives
To assess the effect of planned birth (either by induction of labour or caesarean birth), at or near term (37 to 40 weeks' gestation) compared with an expectant approach for improving health outcomes for women with gestational diabetes and their infants. The primary outcomes relate to maternal and perinatal mortality and morbidity.
Search methods
We searched Cochrane Pregnancy and Childbirth’s Trials Register, ClinicalTrials.gov and the WHO International Clinical Trials Registry Platform (ICTRP) (15 August 2017), and reference lists of retrieved studies.
Selection criteria
We included randomised trials comparing planned birth, at or near term (37 to 40 weeks' gestation), with an expectant approach, for women with gestational diabetes. Cluster‐randomised and non‐randomised trials (e.g. quasi‐randomised trials using alternate allocation) were also eligible for inclusion but none were identified.
Data collection and analysis
Two of the review authors independently assessed study eligibility, extracted data and assessed the risk of bias of the included study. The quality of the evidence was assessed using the GRADE approach.
Main results
The findings of this review are based on a single trial involving 425 women with gestational diabetes. The trial compared induction of labour with expectant management (waiting for the spontaneous onset of labour in the absence of any maternal or fetal issues that may necessitate birth) in pregnant women with gestational diabetes at term. We assessed the overall risk of bias as being low for most domains, apart from performance, detection and attrition bias (for outcome perineum intact), which we assessed as being at high risk. It was an open‐label trial, and women and healthcare professionals were not blinded.
There were no clear differences between women randomised to induction of labour and women randomised to expectant management for maternal mortality or serious maternal morbidity (risk ratio (RR) 1.48, 95% confidence interval (CI) 0.25 to 8.76, one trial, 425 women); caesarean section (RR 1.06, 95% CI 0.64 to 1.77, one trial, 425 women); or instrumental vaginal birth (RR 0.81, 95% CI 0.45 to 1.46, one trial, 425 women). For the primary outcome of maternal mortality or serious maternal morbidity, there were no deaths in either group and serious maternal morbidity related to admissions to intensive care unit. The quality of the evidence contributing to these outcomes was assessed as very low, mainly due to the study having high risk of bias for some domains and because of the imprecision of effect estimates.
In relation to primary neonatal outcomes, there were no perinatal deaths in either group. The quality of evidence for this outcome was judged as very low, mainly due to high risk of bias and imprecision of effect estimates. There were no clear differences in infant outcomes between women randomised to induction of labour and women randomised to expectant management: shoulder dystocia (RR 2.96, 95% CI 0.31 to 28.21, one trial, 425 infants, very low‐quality evidence); large‐for‐gestational age (RR 0.53, 95% CI 0.28 to 1.02, one trial, 425 infants, low‐quality evidence).
There were no clear differences between women randomised to induction of labour and women randomised to expectant management for postpartum haemorrhage (RR 1.17, 95% CI 0.53 to 2.54, one trial, 425 women); admission to intensive care unit (RR 1.48, 95% CI 0.25 to 8.76, one trial, 425 women); and intact perineum (RR 1.02, 95% CI 0.73 to 1.43, one trial, 425 women). No infant experienced a birth trauma, therefore, we could not draw conclusions about the effect of the intervention on the outcomes of brachial plexus injury and bone fracture at birth. Infants of women in the induction‐of‐labour group had higher incidences of neonatal hyperbilirubinaemia (jaundice) when compared to infants of women in the expectant‐management group (RR 2.46, 95% CI 1.11 to 5.46, one trial, 425 women).
We found no data on the following prespecified outcomes of this review: postnatal depression, maternal satisfaction, length of postnatal stay (mother), acidaemia, intracranial haemorrhage, hypoxia ischaemic encephalopathy, small‐for‐gestational age, length of postnatal stay (baby) and cost.
The authors of this trial acknowledge that it is underpowered for their primary outcome of caesarean section. The authors of the trial and of this review note that the CIs demonstrate a wide range, therefore making it inappropriate to draw definite conclusions.
Authors' conclusions
There is limited evidence to inform implications for practice. The available data are not of high quality and lack power to detect possible important differences in either benefit or harm. There is an urgent need for high‐quality trials evaluating the effectiveness of planned birth at or near term gestation for women with gestational diabetes compared with an expectant approach.
Plain language summary
Planned birth at or near term for pregnant women with gestational diabetes and their infants
What is the issue?
The aim of this Cochrane review was to find out if planning an elective birth at or near the term of pregnancy, compared to waiting for labour to start spontaneously, has an impact on the health of women with gestational diabetes and the health of their babies. Planned early birth means either induction of labour or caesarean birth, and 'at or near term' means 37 to 40 weeks' gestation. To answer this question, we collected and analysed all relevant studies conducted up to August 2017.
Why is this important?
Women with gestational diabetes (glucose intolerance arising during pregnancy) and their babies are at increased risk of health complications (e.g. high blood pressure, bigger babies). Because of the complications sometimes associated with birthing a big baby, many clinicians have recommended that women with gestational diabetes have an elective birth (generally an induction of labour) at or near term (37 to 40 weeks' gestation) rather than waiting for labour to start spontaneously, or until 41 weeks' gestation if all is well. Induction has disadvantages of increasing the incidence of forceps or ventouse births, and women often find it difficult to cope with an induced labour. Caesarean section is a major operation which can lead to blood loss, infections and increased chance of problems with subsequent births. Early birth can increase the chance of breathing problems for babies. It is important to know which approach to birth has a better impact on the health outcomes of women with gestational diabetes and their babies.
What evidence did we find?
Our search identified one trial involving 425 women and their babies. In this trial, 214 women had an induction of their labour at term, the other 211 women waited for a spontaneous onset of their labour.
The findings of this trial highlighted no clear difference between the babies of women in either group in relation to the number of large babies, baby's shoulder getting stuck during birth or babies with breathing problems, low blood sugar and admission to a neonatal intensive care unit. No baby in the trial experienced birth trauma. In the group of women whose labour was induced, there were more incidences of jaundice in the babies. There was no clear difference between women in either group in relation to serious health problems for women, caesarean section, instrumental vaginal birth, postpartum haemorrhage, admission to an intensive care unit and intact perineum. There were no reports in either group of maternal deaths. It should be noted that most of the evidence was found to be of very low quality.
The following outcomes were not reported: postnatal depression, maternal satisfaction, length of postnatal stay (mother), babies with high blood acid, bleeding in the baby's brain, other brain problems for the babies, babies small‐for‐gestational age and length of baby's postnatal stay.
What does this mean?
There is insufficient evidence to clearly identify if there are differences in health outcomes for women with gestational diabetes and their babies when elective birth is undertaken compared to waiting for labour to start spontaneously or until 41 weeks' gestation if all is well. More research is needed to answer this question.
Summary of findings
Summary of findings for the main comparison. Planned elective birth (induction or caesarean) compared to expectant management for pregnant women with gestational diabetes.
| Planned birth (induction or caesarean) compared to expectant management for pregnant women with gestational diabetes | ||||||
| Patient or population: pregnant women with gestational diabetes, between 38 ‐ 39 gestational weeks, without other maternal or fetal conditions. Setting: Italy, Slovenia and Israel. Intervention: planned birth (induction of labour at 38/39 gestational weeks). Comparison: expectant management of labour. | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Risk with expectant management | Risk with planned elective birth (induction) | |||||
| Maternal mortality or serious maternal morbidity | 9 per 1000 | 14 per 1000 (2 to 83) | RR 1.48 (0.25 to 8.76) | 425 (1 RCT) | ⊕⊝⊝⊝ VERY LOW 1 2 3 | No events reported in either group for maternal mortality and serious maternal morbidity related to the intensive care unit admissions |
| Caesarean section | 118 per 1000 | 126 per 1000 (76 to 210) | RR 1.06 (0.64 to 1.77) | 425 (1 RCT) | ⊕⊝⊝⊝ VERY LOW 1 2 4 | |
| Instrumental vaginal birth | 104 per 1000 | 84 per 1000 (47 to 152) | RR 0.81 (0.45 to 1.46) | 425 (1 RCT) | ⊕⊝⊝⊝ VERY LOW 1 2 5 | |
| Perinatal mortality rate | 0 per 1000 | 0 per 1000 (0 to 0) | not estimable | 425 (1 RCT) | ⊕⊝⊝⊝ VERY LOW 1 2 6 | No events reported in either group |
| Shoulder dystocia | 5 per 1000 | 14 per 1000 (1 to 134) | RR 2.96 (0.31 to 28.21) | 425 (1 RCT) | ⊕⊝⊝⊝ VERY LOW 1 2 7 | |
| Large‐for‐gestational age | 114 per 1000 | 60 per 1000 (32 to 116) | RR 0.53 (0.28 to 1.02) | 425 (1 RCT) | ⊕⊕⊝⊝ LOW 1 2 8 | |
| Acidaemia ‐ not measured | ‐ | ‐ | ‐ | ‐ | ‐ | |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio; OR: Odds ratio; | ||||||
| GRADE Working Group grades of evidence High quality: We are very confident that the true effect lies close to that of the estimate of the effect Moderate quality: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low quality: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low quality: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | ||||||
1 One study included in this review, therefore inconsistency cannot be assessed
2 High risk of bias for blinding of participants and personnel and blinding of outcome assessment (downgraded x 1)
3 Confidence interval crosses the line of no effect and confidence intervals include appreciable benefit and harm. Criteria for optimal information size (23,171 women per group), in relation to intensive care unit admission, have not been met (downgraded x 2).
4 Confidence interval crosses the line of no effect and confidence intervals include appreciable benefit and harm. Criteria for optimal information size (1,616 women per group) have not been met (downgraded x 2).
5 Confidence interval crosses the line of no effect and confidence intervals include appreciable benefit and harm. Criteria for optimal information size (1,920 women per group) have not been met (downgraded x 2).
6 No events for this outcome. Drawing on the neonatal deaths recorded in Rosenstein 2012 at 37, 38 and 39 weeks' gestation for women with gestational diabetes, criteria for optimal information size (477,500 women per group) have not been met (downgraded x 2).
7 Confidence interval crosses the line of no effect and confidence intervals include appreciable benefit and harm. Criteria for optimal information size (46,145 women per group) have not been met (downgraded x 2).
8 Confidence interval crosses the line of no effect and confidence intervals include appreciable benefit. Criteria for optimal information size (1,743 women per group) have not been met (downgraded x 1).
Background
A planned birth has been advocated in the setting of an otherwise low‐risk singleton pregnancy after 41 weeks (Gulmezoglu 2012); by some healthcare professionals at 37 to 40 weeks of pregnancy where suspicion of macrosomia exists (Boulvain 2016); and for women with an uncomplicated twin pregnancy at 37 weeks' gestation (Dodd 2014). The aim of such an intervention is to reduce adverse pregnancy outcomes associated with these scenarios. Systematic reviews show that induction of labour after 41 weeks is the only intervention associated with a reduction in perinatal mortality (Gulmezoglu 2012). In the area of pregnancy‐related diabetes, a previously published Cochrane review concluded that elective birth at term in pregnant women with insulin‐requiring diabetes reduces the risk of macrosomia but does not impact on maternal or neonatal morbidity (Boulvain 2001). While most women in this prior review had gestational diabetes, it also included several women with pre‐existing Type 2 diabetes.
The original Cochrane review 'Elective delivery in diabetic pregnant women' (Boulvain 2001) has now been split into the following two reviews.
Planned birth at or near term for improving health outcomes for pregnant women with gestational diabetes and their infants (this review).
Planned birth at or near term for improving health outcomes for pregnant women with pre‐existing diabetes and their infants (Biesty 2017a).
As gestational diabetes is typically a transient glucose abnormality occurring late in the second trimester of pregnancy, whilst pre‐existing diabetes exists throughout the entire pregnancy, it is important to clearly differentiate between these different conditions when approaching the issue of planned birth. It is acknowledged that there will be similarities in the background, methods and outcomes between these two systematic reviews.
Description of the condition
Gestational diabetes is defined as carbohydrate intolerance resulting in hyperglycaemia (high blood sugar) of variable severity, with onset or first recognition during pregnancy (WHO 2014). The diagnosis excludes those with diabetes in pregnancy which is likely to represent overt or pre‐existing diabetes. The worldwide prevalence of gestational diabetes varies significantly, but rates are clearly rising in parallel with increasing rates of Type 2 diabetes and obesity (Bottalico 2007). While prevalence is most often reported as 2% to 6% of pregnancies, rates of over 20% are cited in selected populations (Buckley 2012); however, inconsistencies in screening and diagnostic criteria reduce the accuracy of prevalence data (Avalos 2013; Benhalima 2015). A recently published Cochrane review has concluded that there is insufficient evidence to suggest which strategy is best for diagnosing gestational diabetes (Farrar 2017).
Women with gestational diabetes have increased insulin resistance; this can lead to maternal hyperglycaemia and increased glucose transport across the placenta, with resultant fetal hyperinsulinaemia (higher‐than‐normal levels of insulin) and accelerated growth (Setji 2005). Gestational diabetes is associated with an elevated risk of complications during pregnancy including pre‐eclampsia, polyhydramnios (excess amniotic fluid) and the need for caesarean birth (Reece 2010; O’Sullivan 2012). While gestational diabetes typically resolves after the pregnancy, it is associated with an increased lifetime risk of maternal Type 2 diabetes (O’Sullivan 1980; Metzger 1985; O’Dea 2015; Noctor 2016). Infants born to mothers with gestational diabetes are more likely to be macrosomic (birthweight > 4000 g) or large‐for‐gestational age (birthweight above the 90th percentile for gestational age) (Crowther 2005; Metzger 2008; Reece 2010). These infants are at increased risk of birth injury and later‐life Type 2 diabetes and metabolic syndrome (Reece 2010). Additional infant complications include neonatal hypoglycaemia (low blood sugar), respiratory complications and admission to a neonatal intensive care unit (Ramos‐Roman 2011; Kgosidialwa 2015).
During the second and early third trimesters, physiological insulin resistance increases to facilitate glucose transfer across the placenta to the fetus, and ensure adequate growth and development (Farrar 2016). This insulin‐resistant state is created by placental hormones including oestrogen, progesterone, cortisol, placental lactogen, prolactin and growth hormone (Setji 2005). The maternal pancreas compensates for this pregnancy‐induced insulin resistance by secreting more insulin (Wilcox 2005; McCurdy 2010). In gestational diabetes, more severe insulin resistance is accompanied by insufficient release of compensatory insulin, which limits the transport of glucose into cells and increases maternal glucose concentration (Setji 2005). This results in hyperglycaemia in the developing fetus, stimulating fetal insulin production and leading to over‐nourishment of the fetus (Tieu 2008). In recent times, it is becoming evident that additional factors such as alterations in lipid metabolism and inflammatory change may also contribute to the abnormal metabolic environment associated with pregnancies complicated by diabetes, particularly when obesity co‐exists (Catalano 2011).
Gestational diabetes is associated with complications for the infant including fetal macrosomia, being large‐for‐gestational age, and increased need for admission to a neonatal intensive care unit (O’Sullivan 2012; Wendland 2012; Kgosidialwa 2015). A strong emphasis is placed on the association with macrosomia because of consequences such as shoulder dystocia (baby's shoulder getting stuck during birth) and other birth injuries (Tieu 2008; Reece 2010). One systematic review concluded that after the index pregnancy, the cumulative incidence of diabetes ranged from 2.6% to 70% in studies that examined women 6 weeks to 28 years postpartum (Kim 2002). Predictive factors for developing abnormal glucose tolerance after gestational diabetes include glucose levels on the pregnancy oral glucose tolerance test, a family history of diabetes and body mass index at follow‐up (Noctor 2016). In the long‐term, offspring of women with gestational diabetes are at increased risk of obesity, pre‐diabetes, diabetes and neurosensory disabilities including autism spectrum disorders (Krakowiak 2012; Page 2014).
There is a wide range of maternal risk factors associated with the development of gestational diabetes, including maternal obesity, increased maternal age, family history of Type 2 diabetes, and having gestational diabetes or macrosomia in a previous pregnancy (Aktun 2015; Chen 2015; Duman 2015; Jafari‐Shobeiri 2015). Certain ethnicities including Asian, African American, Native American, Hispanic and Pacific Island also have an increased risk of developing gestational diabetes (Carolan 2012; Schneider 2012; Chamberlain 2013; Kim 2013). Despite the large numbers at risk of gestational diabetes, the optimal screening approach is under dispute. Some groups, including the American Diabetes Association (ADA) and the International Association of Diabetes and Pregnancy Study Groups (IADPSG), advocate universal screening using a diagnostic test (75 g oral glucose tolerance test), whereas others, including the National Institute for Health and Care Excellence (NICE) in the UK, recommend risk‐factor based screening (Metzger 2010; NICE 2015; Benhalima 2016; ADA 2017). It must be noted that a significant number of women have no classical risk factors for gestational diabetes, and women falling into this category who were diagnosed on the basis of universal screening experience more adverse pregnancy outcomes than those with normal glucose tolerance (Avalos 2013).
Similar to screening, the diagnosis of gestational diabetes is controversial. The condition is identified by a diagnostic oral glucose tolerance test; however, the amount of glucose recommended for this test differs (75 g to 100 g) and there is significant variation in postprandial glucose concentrations (blood sugar levels after eating) above which gestational diabetes is diagnosed (ACOG 2013; New Zealand Ministry of Health 2014; WHO 2014; NICE 2015). To illustrate further, in 2008 the International Association of Diabetes and Pregnancy Study Groups (IADPSG) developed a consensus statement for a new strategy to diagnose gestational diabetes based on the results of the Hyperglycaemia and Neonatal Outcomes (HAPO) study (Metzger 2008; Metzger 2010). The thresholds for diagnosis chosen, by the IADPSG, are the average glucose values at which odds for birthweight > 90th percentile, cord C‐peptide > 90th percentile and percent body fat > 90th percentile reached 1.75 times the estimated odds of these outcomes at mean glucose levels (IADPSG Consensus Panel 2010). These guidelines were subsequently endorsed by the World Health Organization (WHO), The Endocrine Society and the International Federation of Gynecology and Obstetrics (FIGO) (Blumer 2013; WHO 2014; Hod 2015). However, for a variety of reasons, including health economic analysis and treatment effects from intervention trials, these recommendations have not been adopted universally (Bilous 2015; NICE 2015). Finally, while gestational diabetes is typically diagnosed between 24 and 28 weeks' gestation, earlier assessment is often advised for women considered to be high‐risk (Setji 2005). Unfortunately, there are no randomised controlled trial data examining the balance between the additional benefits and the cost of detecting and treating women diagnosed with gestational diabetes (excluding those with overt diabetes) in early pregnancy (McIntyre 2016).
Description of the intervention
Following diagnosis, the primary aims of treatment for gestational diabetes are to optimise glycaemic control and improve pregnancy outcomes (Brown 2016; Brown 2017a; Brown 2017b). Women are typically treated with diet and lifestyle advice along with self‐monitoring of glucose to ensure tight glycaemic goals are achieved (NICE 2015). If these interventions cannot limit maternal hyperglycaemia, pharmacological therapy is introduced. Increased obstetric monitoring, including more frequent antenatal visits and regular ultrasound monitoring, is typically employed to monitor fetal growth and other potential comorbidities in women with gestational diabetes. In the United Kingdom, the NICE guidelines advise women with gestational diabetes to birth no later than 40+6 weeks' (40 weeks plus six days') gestation and to consider elective birth before 40+6 weeks if there are maternal or fetal complications (NICE 2015). Guidelines issued by the Americian College of Obstetricians and Gynecologists (ACOG) in July 2017 suggest that women with gestational diabetes treated with diet and lifestyle advice should be offered expectant management up to 40+6 weeks' gestation; women on medications who are achieving their glycaemic goals should birth between 39 and 39+6 weeks' gestation; and women with "poorly controlled" glycaemic goals should birth between 37 and 38+6 weeks' gestation (ACOG 2017).
A woman’s pregnancy is considered to be 'at term' when her pregnancy duration reaches 37 weeks (Gulmezoglu 2012). Planned birth involves the early birth of the infant either by induction of labour or by caesarean section. This typically takes place between 37 and 40 weeks' gestation. Methods of induction vary according to local protocols and typically depend on cervical status. The process generally involves cervical ripening with misoprostol or prostaglandin E2 (PGE2) followed by amniotomy (the artificial rupture of membranes) and oxytocin infusion if labour has not started (Boulvain 2016). The alternative is the expectant approach to the management of birth, which refers to waiting for the spontaneous onset of labour in the absence of any maternal or fetal issues that may necessitate birth (Bond 2017).
How the intervention might work
In women with gestational diabetes, the rationale for performing an elective birth includes possible reduction in perinatal complications, especially those related to macrosomia (Brudenell 1989). Macrosomia is typically defined as a birthweight of more than 4000 g (Feig 2015). It is associated with an increased chance of prolonged labour, maternal trauma, emergency caesarean birth and a higher risk of birth injuries for the infant, including clavicle fracture and brachial plexus injury (Perlow 1996; Ju 2009). However, for some women and babies the birth can be straightforward.
A recent Cochrane review of induction of labour at or near term for suspected fetal macrosomia (Boulvain 2016) concluded that further trials are necessary to clarify if the benefits, including lower mean birthweight and fewer instances of birth fracture and shoulder dystocia, outweigh the risks which include increased perineal damage.
Why it is important to do this review
In 1989, the St Vincent declaration called on governments and healthcare services to implement effective measures to achieve pregnancy outcomes in women with diabetes that approximate those of women without diabetes within five years (St Vincent Declaration 1990). While this goal was not achieved, it is important that we strive to identify any measures that may assist in meeting this target in our care for the increasing numbers of women with gestational diabetes. Planned births may have potential benefits, possibly reducing the risks of prolonged labour and elevated rates of caesarean section following induction of labour (Macer 1992). Birth by caesarean section, including elective caesarean, may increase the risk of maternal morbidity including postpartum infections, haemorrhage or uterine rupture during subsequent labour (Irion 1998). Induction of labour may lead to increased interventions during labour and birth and an increase in maternal morbidity (Khireddine 2013). Furthermore, early‐term birth is associated with an increased risk of multiple neonatal morbidities including respiratory distress syndrome and the need for mechanical ventilation and admission to a neonatal intensive care unit (ACOG 2013). Women’s views on elective birth versus continued antenatal surveillance should also be considered (Dodd 2014). The existing Cochrane review on this topic, ‘Elective delivery in diabetic pregnant women’ (Boulvain 2001) includes women with insulin‐treated diabetes who had a diagnosis of gestational diabetes or Type 2 diabetes, and does not examine women with gestational diabetes treated with non‐pharmacological interventions alone. Furthermore, this review was published in 2001 and it is possible that additional evidence on this subject is now available for analysis.
Based on the above, it is now important to assess the effect of a policy of planned birth compared with an expectant approach on maternal and perinatal mortality and morbidity in women with gestational diabetes. Women and healthcare professionals need unbiased information on this subject and this is best provided by meta‐analysis of high‐quality randomised controlled trials.
Objectives
To assess the effect of planned birth (either by induction of labour or caesarean birth), at or near term (37 to 40 weeks' gestation) compared with an expectant approach for improving health outcomes for women with gestational diabetes and their infants. The primary outcomes relate to maternal and perinatal mortality and morbidity.
Methods
Criteria for considering studies for this review
Types of studies
We included all published randomised trials (including those using a cluster‐randomised design) and non‐randomised trials which compared planned birth at or near term gestation, with an expectant approach for women with gestational diabetes. Non‐randomised trials are trials in which participants are allocated to treatment groups using non‐random methods (e.g. alternate) (EPOC 2016).
Cross‐over studies were excluded as this design is not appropriate for this intervention.
Studies published in abstract form were only eligible for inclusion where information on risk of bias and primary or secondary outcomes could be obtained.
Types of participants
Pregnant women, at or near term gestation (37 to 40 weeks' gestation), with gestational diabetes as diagnosed according to each included study.
Pregnant women with pre‐existing diabetes will be included in a different Cochrane review, titled 'Planned birth at or near term for improving health outcomes for pregnant women with pre‐existing diabetes and their infants' (Biesty 2017a).
We planned to exclude trials that included women both with gestational diabetes and pre‐existing diabetes where data could not be separated.
Types of interventions
Planned birth (induction of labour or caesarean section) at or near term gestation.
Induction of labour was defined by trial authors and may include the use of prostaglandins, misoprostol, oxytocin, amniotomy or a combination of these.
Comparisons
Planned birth at or near term gestation versus an expectant approach
An expectant approach to the management of birth refers to waiting for the spontaneous onset of labour in the absence of any maternal of fetal issues that may necessitate birth (Bond 2017) (or until 41 weeks' gestation or more, when induction of labour may be offered).
Types of outcome measures
For this review, we adapted the core outcome set agreed by consensus between review authors of the Cochrane Pregnancy and Childbirth systematic reviews for prevention and treatment of gestational diabetes and pre‐existing diabetes. The core outcome set was adapted to ensure that the outcome measures included were appropriate for this research question.
Primary outcomes
Maternal
Maternal mortality or serious maternal morbidity (i.e. cardiac arrest, respiratory arrest, admission to intensive care unit (ICU))
Caesarean section
Instrumental vaginal birth (forceps or vacuum)
Neonatal
Perinatal mortality rate (corrected, i.e. stillbirths and neonatal deaths excluding lethal congenital anomalies)
Shoulder dystocia
Large‐for‐gestational age (birthweight greater than the 90th centile or as defined by the trial authors)
Acidaemia (as evident by a pH of less than 7.0 or a base deficit greater than 12 mmol/L in umbilical arterial cord blood or neonatal blood sample within the first hour of life, or both)
Where available, all primary outcomes were included in a 'Summary of findings' table.
Secondary outcomes
Maternal
Maternal death
Cardiac arrest
Respiratory arrest
Admission to ICU
Intact perineum
Uterine rupture
Postpartum haemorrhage (defined as 1000 mL or more)
Postnatal depression (as measured by either the Edinburgh Postnatal Depression Scale, the Postpartum Depression Screening Scale, the Beck Depression Inventory or other validated scales)
Maternal satisfaction (as measured by trial authors)
Neonatal
Brachial plexus injury
Bone fracture at birth
Intracranial haemorrhage (all grades)
Hypoxic ischaemic encephalopathy
Respiratory distress syndrome
Neonatal hypoglycaemia (blood glucose concentrations below the normal range, investigator defined)
Neonatal hyperbilirubinaemia (blood bilirubin concentrations above the normal range, investigator defined)
Small‐for‐gestational age (birthweight below the third centile or as defined by the trial authors)
Admission to neonatal ICU
Neurosensory disability (defined by a standardised assessment tool at approximately two years of age)
Health service outcomes
Length of postnatal stay (mother)
Length of postnatal stay (baby)
Cost
Search methods for identification of studies
The following methods section of this review is based on a standard template used by Cochrane Pregnancy and Childbirth.
Electronic searches
We searched Cochrane Pregnancy and Childbirth’s Trials Register by contacting their Information Specialist (15 August 2017).
The Register is a database containing over 23,000 reports of controlled trials in the field of pregnancy and childbirth. For full search methods used to populate Pregnancy and Childbirth’s Trials Register, including the detailed search strategies for CENTRAL, MEDLINE, Embase and CINAHL; the list of handsearched journals and conference proceedings; and the list of journals reviewed via the current awareness service, please follow this link to the editorial information about Cochrane Pregnancy and Childbirth in the Cochrane Library and select the 'Specialized Register' section from the options on the left side of the screen.
Briefly, Cochrane Pregnancy and Childbirth’s Trials Register is maintained by their Information Specialist and contains trials identified from:
monthly searches of the Cochrane Central Register of Controlled Trials (CENTRAL);
weekly searches of MEDLINE (Ovid);
weekly searches of Embase (Ovid);
monthly searches of CINAHL (EBSCO);
handsearches of 30 journals and the proceedings of major conferences;
weekly current awareness alerts for a further 44 journals plus monthly BioMed Central email alerts.
Two people screen the search results and review the full text of all relevant trial reports identified through the searching activities described above. Based on the intervention described, each trial report is assigned a number that corresponds to a specific Pregnancy and Childbirth review topic (or topics), and is then added to the Register. The Information Specialist searches the Register for each review using this topic number rather than keywords. This results in a more specific search set that has been fully accounted for in the relevant review sections (Included studies; Excluded studies; Studies awaiting classification).
In addition, we searched ClinicalTrials.gov and the WHO International Clinical Trials Registry Platform (ICTRP) (15 August 2017) for unpublished, planned and ongoing trial reports using the search methods detailed in Appendix 1.
Searching other resources
We searched the reference lists of retrieved studies.
We did not apply any language or date restrictions.
Data collection and analysis
We designed a form to extract data. Two review authors (LB and DD) independently extracted the data from eligible studies using the agreed form. We did not have any disagreements but if we had, we would have resolved these through discussion or, if required, through consultation with a third person. We pilot tested the data extraction tool on one related, but not included, paper prior to conducting the full review and amended the form as necessary.
Where additional information was needed, we contacted the authors of the original report to provide further details and have noted this contact in Characteristics of included studies.
Selection of studies
Two review authors independently assessed for inclusion all potentially eligible studies identified by our search strategy. We planned to resolve any disagreement through discussion or by consulting a third person if necessary.
We created a study flow diagram to map out the number of records identified, included and excluded (Figure 1).
1.
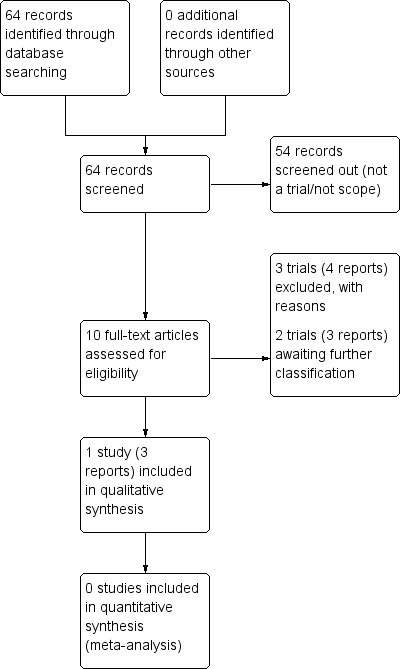
Study flow diagram.
Data extraction and management
We designed a form to extract data. For eligible studies, two review authors extracted the data independently using the agreed form. We did not have any disagreements but if we had, we would have resolved discrepancies through discussion or, if required, through consultation with a third person. We pilot tested the data extraction tool on one related, but not included, paper prior to conducting the full review and amended the form as necessary. LB entered all data into Review Manager 5 (RevMan 5) software (RevMan 2014) which was checked for accuracy by DD.
Where additional information was needed, we contacted the authors of the original report to provide further details and have noted this contact in Characteristics of included studies.
Assessment of risk of bias in included studies
Two review authors (LB and DD) independently assessed the risk of bias in each study using the criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We had no disagreements but if we had, we would have resolved these through discussion or by consultation with a third person if necessary.
We did not identify any cluster‐randomised trials. Had we done so, we would have used appropriate methods for assessing bias in these designs as outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011).
Where information on risk of bias relates to unpublished data or correspondence with trialists, this is noted in the 'Risk of bias' table.
(1) Random sequence generation (checking for possible selection bias)
We described the method used to generate the allocation sequence in sufficient detail to allow an assessment of whether it should produce comparable groups.
For each included study we assessed the method as being at:
low risk of bias (any truly random process, e.g. random number table; computer random number generator);
high risk of bias (any non‐random process, e.g. odd or even date of birth; hospital or clinic record number);
unclear risk of bias.
(2) Allocation concealment (checking for possible selection bias)
For each included study we described the method used to conceal allocation to interventions prior to assignment and assessed whether intervention allocation could have been foreseen in advance of, or during recruitment, or changed after assignment.
We assessed the methods as being at:
low risk of bias (e.g. telephone or central randomisation; consecutively numbered sealed opaque envelopes);
high risk of bias (open random allocation; unsealed or non‐opaque envelopes; alternation; date of birth);
unclear risk of bias.
(3.1) Blinding of participants and personnel (checking for possible performance bias)
For each included study we described the methods used, if any, to blind study participants and personnel from knowledge of which intervention a participant received. We considered that studies were at low risk of bias if they were blinded, or if we judged that the lack of blinding was unlikely to affect results.
We assessed the methods as being at:
low, high or unclear risk of bias for participants;
low, high or unclear risk of bias for personnel.
(3.2) Blinding of outcome assessment (checking for possible detection bias)
For each included study we described the methods used, if any, to blind outcome assessors from knowledge of which intervention a participant received.
We assessed methods used to blind outcome assessment as being at low, high or unclear risk of bias.
(4) Incomplete outcome data (checking for possible attrition bias due to the amount, nature and handling of incomplete outcome data)
For each included study, and for each outcome or class of outcomes, we described the completeness of data including attrition and exclusions from the analysis. We stated whether attrition and exclusions were reported and the numbers included in the analysis at each stage (compared with the total randomised participants), reasons for attrition or exclusion where reported, and whether missing data were balanced across groups or were related to outcomes. Where sufficient information was reported, or could be supplied by the trial authors, we planned to re‐include missing data in the analyses that we undertook.
We assessed methods as being at:
low risk of bias (e.g. no missing outcome data; missing outcome data balanced across groups; the proportion of missing data was less than the effect size, and so unlikely to overturn the study result);
high risk of bias (i.e. where attrition is 20% or more for outcomes or groups of outcomes). We explored if number or reasons for missing data were balanced across groups);
unclear risk of bias.
(5) Selective reporting (checking for reporting bias)
For each included study we described how we investigated the possibility of selective outcome reporting bias and what we found.
We assessed the methods as being at:
low risk of bias (where it was clear that all of the study’s prespecified outcomes and all expected outcomes of interest to the review have been reported);
high risk of bias (where not all the study’s prespecified outcomes were reported; one or more reported primary outcomes were not prespecified; outcomes of interest were reported incompletely and so could not be used; study failed to include results of a key outcome that would have been expected to have been reported);
unclear risk of bias.
(6) Other bias (checking for bias due to problems not covered by (1) to (5) above)
For each included study we described any important concerns we had about other possible sources of bias.
(7) Overall risk of bias
We made explicit judgements about whether studies were at high risk of bias, according to the criteria given in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). With reference to (1) to (6) above, we planned to assess the likely magnitude and direction of the bias and whether we considered it was likely to have an impact on the findings. In future updates, we will explore the impact of the level of bias through undertaking Sensitivity analysis.
Assessment of the quality of the evidence using the GRADE approach
We used the GRADE approach as outlined in the GRADE handbook to assess the quality of the body of evidence relating to the following outcomes for the comparisons of planned birth (induction of labour or caesarean section), at or near term gestation versus an expectant approach.
Maternal mortality or serious maternal morbidity (e.g. cardiac arrest, respiratory arrest, admission to ICU)
Caesarean section
Instrumental vaginal birth (forceps or vacuum)
Perinatal mortality rate (corrected, i.e. stillbirths and neonatal deaths excluding lethal congenital anomalies)
Shoulder dystocia
Large‐for‐gestational age (birthweight greater than the 90th centile or as defined by the trial authors)
Acidaemia (as evident by a pH of less than 7.0, or a base deficit greater than 12 mmol/L in umbilical arterial cord blood or neonatal blood sample within the first hour of life, or both)
We used GRADEpro Guideline Development Tool software to import data from RevMan 5 and create 'Summary of findings' tables. A summary of the intervention effect and a measure of quality for each of the above outcomes was produced using the GRADE approach. The GRADE approach uses five considerations (study limitations, consistency of effect, imprecision, indirectness and publication bias) to assess the quality of the body of evidence for each outcome. The evidence can be downgraded from 'high quality' by one level for serious limitations (or by two levels for very serious limitations) depending on assessments for risk of bias, indirectness of evidence, serious inconsistency, imprecision of effect estimates or potential publication bias.
Measures of treatment effect
Dichotomous data
For dichotomous data, we present results as summary risk ratios with 95% confidence intervals.
Continuous data
We did not find any continuous data but should we find such data in future updates of the review, we will use the mean difference if outcomes are measured in the same way between trials. We will use the standardised mean difference to combine data from trials that measure the same outcome, but use different methods.
Unit of analysis issues
Cluster‐randomised trials
We did not find any cluster‐randomised trials. Had we done so we would have considered them for inclusion in the analyses along with individually randomised trials. In future updates of this review, we will consider including cluster trials and will adjust their sample sizes using the methods described in Cochrane Handbook (Higgins 2011) using an estimate of the intracluster correlation co‐efficient (ICC) derived from the trial (if possible), from a similar trial, or from a study of a similar population. If we use ICCs from other sources, we will report this and conduct sensitivity analyses to investigate the effect of variation in the ICC. If we identify both cluster‐randomised trials and individually‐randomised trials, we plan to synthesise the relevant information. We will consider it reasonable to combine the results from both if there is little heterogeneity between the study designs and the interaction between the effect of intervention and the choice of randomisation unit is unlikely.
We will also acknowledge heterogeneity in the randomisation unit and perform a sensitivity analysis to investigate the effects of the randomisation unit.
Studies with multiple arms
We did not find any studies with multiple arms. In future updates, for studies with multiple treatment arms, we will combine all relevant experimental intervention groups in the study (e.g. groups with different methods for induction of labour) into a single group, and combine all comparable relevant control intervention groups into a single control group and perform a single pair‐wise comparison, as recommended in the Cochrane Handbook (Higgins 2011).
Dealing with missing data
For included studies, we noted levels of attrition. We had planned to explore the impact of including studies with high levels of missing data in the overall assessment of treatment effect by using sensitivity analysis but this was not possible because of inclusion of only one study.
For all outcomes, we carried out analyses, as far as possible, on an intention‐to‐treat basis, i.e. we included all participants randomised to each group in the analyses, and all participants were analysed in the group to which they were allocated, regardless of whether they received the allocated intervention. The denominator for each outcome in each trial was the number randomised minus any participants whose outcomes are known to be missing.
Assessment of heterogeneity
Because we could only include one study, there was no heterogeneity to assess. In future updates, we will assess statistical heterogeneity in each meta‐analysis using the Tau2, I2 and Chi2 statistics. We will regard heterogeneity as substantial if I2 is greater than 30% and either Tau2 is greater than zero, or there is a low P value (less than 0.10) in the Chi2 test for heterogeneity.
Assessment of reporting biases
Because we could only include one study, we did not assess reporting biases. In future updates, if there are 10 or more studies in the meta‐analysis we will investigate reporting biases (such as publication bias) using funnel plots. We will assess funnel plot asymmetry visually. If asymmetry is suggested by a visual assessment, we will perform exploratory analyses to investigate it.
Data synthesis
We carried out statistical analysis using the RevMan 5 (RevMan 2014). We did not conduct a meta‐analysis. In future updates, we will use fixed‐effect meta‐analysis for combining data where it is reasonable to assume that studies are estimating the same underlying treatment effect, i.e. where trials are examining the same intervention, and the trials’ populations and methods are judged sufficiently similar. If there is clinical heterogeneity sufficient to expect that the underlying treatment effects differ between trials, or if substantial statistical heterogeneity is detected, we will use random‐effects meta‐analysis to produce an overall summary if an average treatment effect across trials is considered clinically meaningful. The random‐effects summary will be treated as the average of the range of possible treatment effects and we will discuss the clinical implications of treatment effects differing between trials. If the average treatment effect is not clinically meaningful we will not combine trials.
If we use random‐effects analyses, the results will be presented as the average treatment effect with 95% confidence intervals, and the estimates of Tau2 and I2.
Subgroup analysis and investigation of heterogeneity
We had planned to investigate substantial heterogeneity using subgroup analyses and sensitivity analyses, but were unable to do so due to there being only one included study. In future updates we will explore heterogeneity and consider whether an overall summary is meaningful, and if it is, we will use random‐effects analysis to produce it.
In future updates, we plan to carry out the following subgroup analyses.
Parity: primiparous women versus multiparous women
Birth by planned caesarean section versus planned induction of labour
The following outcomes will be used in subgroup analysis.
Maternal
Maternal mortality or serious maternal morbidity (e.g. cardiac arrest, respiratory arrest, admission to ICU)
Caesarean section
Instrumental vaginal birth (forceps or vacuum)
Neonatal
Corrected perinatal mortality rate (stillbirth and early neonatal deaths, excluding lethal congenital anomalies)
Shoulder dystocia
Large‐for‐gestational age (birthweight greater than the 90th centile or as defined by the trial authors)
Acidaemia (as evident by a pH of less than 7.0, or a base deficit greater than 12 mmol/L in umbilical arterial cord blood or neonatal blood sample within the first hour of life, or both.
We will assess subgroup differences by interaction tests available within RevMan 5 (RevMan 2014). We will report the results of subgroup analyses quoting the Chi2 statistic and P value, and the interaction test I2 value.
Sensitivity analysis
We had planned to conduct a sensitivity analysis on risk of bias in trials by excluding all studies at high or unclear risk of bias for either sequence generation or allocation concealment, based on growing empirical evidence that these factors are particularly important potential sources of bias (Higgins 2011). We had also planned to limit sensitivity analyses to primary outcomes. However, because we included only one study, it was not possible to conduct this sensitivity analysis. In future updates, we will perform this analysis if possible.
Results
Description of studies
Results of the search
See: Figure 1.
Our search strategy identified 64 citations. After screening, we retrieved 10 reports of six trials for potential inclusion. Of those, one trial that included a total of 425 women was included (Alberico 2017; see Characteristics of included studies). We are awaiting the classification of two trials (Henry 1992; Dhaneshwor 2011 (CTRI/2011/12/002290)) see Characteristics of studies awaiting classification). Three trials (Khojandi 1974; Ghosh 1979; Worda 2017) were excluded (see Characteristics of excluded studies).
Included studies
Design
The included study (Alberico 2017) was a multi‐centre open‐labelled randomised controlled trial.
Sample sizes
Four‐hundred and twenty‐five women participated in the trial (Alberico 2017).
Setting
The multi‐centre trial occurred in eight 'centres' in three countries (Italy, Slovenia and Israel). Recruitment took place from March 2010 to March 2014.
Participants
In total, 425 women were randomised (n = 214 intervention arm, n = 211 control arm). Inclusion criteria were: pregnant women, older than 18, with singleton pregnancy, vertex presentation and diagnoses of gestational diabetes in this pregnancy. Women were excluded if they had a diagnosis of overt diabetes, prior caesarean section, obstetrical contraindications to vaginal birth, maternal pregnancy‐related disease or non‐reassuring fetal well‐being necessitating prompt birth, uncertain gestational age, known fetal anomaly, Bishop score less than seven, or estimated fetal weight of 4000 g at enrolment (between 38+0 and 39+0 gestational weeks).
Interventions and comparisons
Women allocated to the to the induction of labour group (n = 214) were admitted to “the ward” between 38+0 and 39+0 gestational weeks and induction of labour was performed using dinoprostone (2 mg vaginally) or dinoprostone (0.5 mg intracervically) in six‐ to eight‐hour intervals (up to five doses), or a dinoprostone (10 mg) vaginal device. Once a woman’s Bishop score exceeded seven, or regular contractions were diagnosed, women were transferred to the delivery ward for an artificial rupture of membranes or oxytocin augmentation. Women for whom cervical ripening did not occur (Bishop score less than seven) after five attempts with prostaglandin E2, were offered either oxytocin or Foley catheter induction or caesarean section. Women allocated to expectant management (n = 211) were followed up twice weekly from 38+0 or 39+0 gestational weeks. Twice weekly they had "electronic fetal heart rate monitoring and a biophysical profile". The authors define this as "intensive follow up". At 41 gestational weeks, women were offered an induction of labour.
Outcomes
Outcomes considered in the review and reported in or extracted from the study included: maternal mortality rate, serious maternal morbidity, caesarean section, instrumental birth, intact perineum, postpartum haemorrhage, admission to intensive care unit, perinatal mortality rate, shoulder dystocia, large‐for‐gestational age, birth trauma, respiratory distress syndrome, neonatal hypoglycaemia, neonatal hyperbilirubinaemia and admission to neonatal intensive care unit.
Funding
The Institute for Maternal & Child Health, IRCCS, Burlo Garofolo "offered human and financial resources to carry out the project". Trial authors’ declaration of interest: none declared.
Excluded studies
We excluded three studies: Ghosh 1979; Khojandi 1974 and Worda 2017 (see Characteristics of excluded studies). Ghosh 1979 was excluded as this study focused on women with "Type B diabetes" (Type 2) and no further details or publications have been made available in relation to this study since the publication of the conference abstract 38 years ago. We excluded Khojandi 1974 because insufficient information was provided to determine randomisation in this study (given that the study was published 42 years ago, we did not attempt to contact the authors). We excluded Worda 2017 as this trial randomised women to either induction of labour at 38 weeks' gestation or 40 weeks' gestation ‐ these intervention groups were not compared with an expectant approach.
Studies awaiting classification
Two studies (Henry 1992; Dhaneshwor 2011 (CTRI/2011/12/002290)) are awaiting classification. We contacted the trial authors of the registered protocols of Dhaneshwor 2011 (CTRI/2011/12/002290) to obtain information in relation to the study, including the status of the study, the findings, and potential publications. We contacted the contact author of Henry 1992 to obtain data that related specifically to the women with gestational diabetes in their study. We have yet to receive replies to our queries (see Characteristics of studies awaiting classification).
Risk of bias in included studies
See and Figure 2 and Figure 3 for a summary of 'Risk of bias' assessment.
2.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
3.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
Allocation
We judged the study to be at low risk of bias for both sequence generation and allocation concealment. The method of randomisation was a computer‐generated list and the list was blocked and stratified by centre. Allocation concealment was achieved through use of consecutively numbered, opaque, sealed envelopes.
Blinding
We judged Alberico 2017 to have a high risk of performance bias given that it was not possible to blind either the women or healthcare professionals to the intervention of induction of labour. We also judged the study to have a high risk of detection bias as the authors report that women’s group assignment was indicated in the paper questionnaire used for the initial reporting of data.
Incomplete outcome data
We grouped all maternal outcomes other than intact perineum and considered Alberico 2017 to have a low risk of bias for attrition in this instance. For the outcome intact perineum, data are available for 94% of the women in the induction of labour group and 82% of women in the expectant management group. Due to imbalances in group attrition, we judged this to be of high risk of bias.
For hyperbilirubinaemia, data are available in the paper for 93% of the newborn infants but it is not identified from which group the missing infants are from. We contacted the authors about this and in correspondence they indicated that data for 14 babies of women in the induction of labour group and 14 in the expectant management group were not available. Therefore, data on this outcome are available for 93.45% of babies of women in the induction of labour group and 92% in the expectant management group. We therefore considered all neonatal outcomes as low risk of bias for attrition bias.
Selective reporting
The published and registered protocol for Alberico 2017 is available (Maso 2011; NCT01058772). All outcomes stated in the protocol, other than neonatal Apgar score at 10 minutes and uterine rupture, are reported in the paper. (The study authors were contacted and provided additional information in relation to uterine rupture and Apgar scores at 10 minutes. They also provided further information in relation to the group allocation for missing data of neonatal hyperbilirubinaemia.)
Other potential sources of bias
We did not identify any additional potential sources of bias in Alberico 2017 so therefore judged this to be of low risk of bias.
Effects of interventions
See: Table 1
Planned birth, at or near term, versus expectant management for pregnant women with gestational diabetes
Primary outcomes
Maternal
There was no clear difference between women randomised to induction of labour and women randomised to expectant management in:
maternal mortality or serious maternal morbidity (risk ratio (RR) 1.48, 95% confidence interval (CI) 0.25 to 8.76, one trial, 425 women, Analysis 1.1);
caesarean section (RR 1.06, 95% CI 0.64 to 1.77, one trial, 425 women, Analysis 1.2);
instrumental vaginal birth (RR 0.81, 95% CI 0.45 to 1.46, one trial, 425 women, Analysis 1.3).
1.1. Analysis.
Comparison 1 Planned elective birth (induction or caesarean) versus expectant, Outcome 1 Maternal mortality or serious maternal morbidity.
1.2. Analysis.
Comparison 1 Planned elective birth (induction or caesarean) versus expectant, Outcome 2 Caesarean section.
1.3. Analysis.
Comparison 1 Planned elective birth (induction or caesarean) versus expectant, Outcome 3 Instrumental vaginal birth.
For the primary outcome of maternal mortality or serious maternal morbidity, there were no deaths in either group and no serious maternal morbidity related to admissions to intensive care unit.
We assessed the quality of the evidence contributing to these outcomes as very low, mainly due to high risk of bias of the included study and imprecision of effect estimates (Table 1).
Neonatal
There were no perinatal deaths in either group (Analysis 1.4) and the quality of evidence for this outcome was judged as very low mainly due to high risk of bias of the included study and imprecision of effect estimates (Table 1) There was no clear difference in infant outcomes between women randomised to induction of labour and women randomised to expectant management in:
1.4. Analysis.
Comparison 1 Planned elective birth (induction or caesarean) versus expectant, Outcome 4 Perinatal mortality rate.
shoulder dystocia (RR 2.96, 95% CI 0.31 to 28.21, one trial, 425 infants, Analysis 1.5, very low‐quality evidence);
large‐for‐gestational age (RR 0.53, 95% CI 0.28 to 1.02, one trial, 425 infants, Analysis 1.6, low‐quality evidence) (Table 1).
1.5. Analysis.
Comparison 1 Planned elective birth (induction or caesarean) versus expectant, Outcome 5 Shoulder dystocia.
1.6. Analysis.
Comparison 1 Planned elective birth (induction or caesarean) versus expectant, Outcome 6 Large‐for‐gestational age.
Secondary outcomes
Maternal
There were no maternal deaths in either group (Analysis 1.7). There was no clear difference between women randomised to induction of labour and women randomised to expectant management in:
1.7. Analysis.
Comparison 1 Planned elective birth (induction or caesarean) versus expectant, Outcome 7 Maternal death.
postpartum haemorrhage (RR 1.17, 95% CI 0.53 to 2.54, one trial, 425 women, Analysis 1.11);
admission to intensive care unit (RR 1.48, 95% CI 0.25 to 8.76, one trial, 425 women, Analysis 1.8).
1.11. Analysis.
Comparison 1 Planned elective birth (induction or caesarean) versus expectant, Outcome 11 Postpartum haemorrhage.
1.8. Analysis.
Comparison 1 Planned elective birth (induction or caesarean) versus expectant, Outcome 8 Admission to ICU.
There was no clear difference between groups for the outcome intact perineum (RR 1.02, 95% CI 0.73 to 1.43, one trial 373 women, Analysis 1.9). It should be noted that data in relation to this outcome were available for 94% of women in the induction‐of‐labour group and 82% of women in the expectant‐management group.
1.9. Analysis.
Comparison 1 Planned elective birth (induction or caesarean) versus expectant, Outcome 9 Intact perineum.
Uterine rupture was listed as a prespecified outcome in the published and registered protocol of the Alberico study (Maso 2011; NCT01058772), but not reported in the study findings. However, we contacted the authors in relation to this and they informed us that no woman participating in the study experienced uterine rupture (Analysis 1.10).
1.10. Analysis.
Comparison 1 Planned elective birth (induction or caesarean) versus expectant, Outcome 10 Uterine rupture.
Neonatal
No infant experienced birth trauma, therefore, we could not draw conclusions about the effect of the intervention on the outcomes of brachial plexus injury and bone fracture at birth (Analysis 1.12; Analysis 1.13).
1.12. Analysis.
Comparison 1 Planned elective birth (induction or caesarean) versus expectant, Outcome 12 Brachial plexus injury.
1.13. Analysis.
Comparison 1 Planned elective birth (induction or caesarean) versus expectant, Outcome 13 Bone fracture at birth.
There was no clear difference in infant outcomes between women randomised to induction of labour and women randomised to expectant management in:
respiratory distress syndrome (RR 1.48, 95% CI 0.25 to 8.76, one trial, 425 infants, Analysis 1.14);
neonatal hypoglycaemia (RR 0.74, 95% CI 0.26 to 2.09, one trial, 425 infants, Analysis 1.15);
and admission to neonatal intensive care unit (RR 0.99, 95% CI 0.14 to 6.94, one trial, 425 infants, Analysis 1.17).
1.14. Analysis.
Comparison 1 Planned elective birth (induction or caesarean) versus expectant, Outcome 14 Respiratory distress syndrome.
1.15. Analysis.
Comparison 1 Planned elective birth (induction or caesarean) versus expectant, Outcome 15 Neonatal hypoglycaemia.
1.17. Analysis.
Comparison 1 Planned elective birth (induction or caesarean) versus expectant, Outcome 17 Admission to NICU.
Infants of women in the induction of labour group had higher incidences of neonatal hyperbilirubinaemia when compared to infants of women in the expectant management group (RR 2.46, 95% CI 1.11 to 5.46, one trial, 397 infants, Analysis 1.16). However it should be noted that the confidence interval is wide, suggesting imprecision.
1.16. Analysis.
Comparison 1 Planned elective birth (induction or caesarean) versus expectant, Outcome 16 Neonatal hyperbilirubinaemia.
Outcomes not reported
Data were not reported, for the following outcomes prespecified in this review.
Maternal
Postnatal depression
Maternal satisfaction
Length of postnatal stay (mother)
Infant
Acidaemia, intracranial haemorrhage
Hypoxia ischaemic encephalopathy
Small‐for‐gestational age
Length of postnatal stay (baby)
There was no information on cost reported by the Alberico 2017 study.
Discussion
Summary of main results
The aim of this review was to assess the effect of planned birth, at or near term (37 to 40 weeks' gestation) compared with an expectant approach, for women with gestational diabetes. The main outcomes relate to maternal and perinatal mortality and morbidity. We included only one trial in this review; therefore, it was not possible to conduct a meta‐analysis.
The included study (Alberico 2017) evaluated maternal and perinatal outcomes after induction of labour versus expectant management in pregnant women with gestational diabetes at term. A total of 425 women participated in this study, which the authors acknowledge is underpowered for their primary outcome of caesarean section. The authors of the study and of this review note that the confidence intervals demonstrate a wide range, therefore making it inappropriate to draw definite conclusions.
Maternal mortality or serious maternal morbidity or incidence of perinatal mortality rates (perinatal deaths) were outcomes reported in Alberico 2017. There were no maternal or perinatal deaths reported in either group and no differences were found for serious maternal morbidity, defined as admissions to intensive care unit. There were also no differences between groups for caesarean section or instrumental vaginal birth. The incidence of caesarean section was 12.6% among women in the induction‐of‐labour group and 11.8% in women in the expectant‐management group. The most common indications for caesarean section were non‐reassuring fetal heart monitoring (25% induction of labour group versus 48% expectant management group), mechanical dystocia, defined as fetopelvic disproportion (33% induction of labour group versus 20% expectant management group), and dynamic dystocia, defined as 'inadequate cervical dilation or fetal descent' (29.6% induction of labour group versus 12% expectant management group). No clear differences were observed for other outcomes in women such as postpartum haemorrhage or intact perineum.
The neonatal data recorded in the Alberico 2017 study identified a difference between infants of women in the induction of labour group versus expectant management, for one outcome — hyperbilirubinaemia. Infants of women in the induction‐of‐labour group had higher incidences of neonatal hyperbilirubinaemia when compared to infants of women in the expectant management group.
No clear difference was noted in shoulder dystocia, with three events of dystocia recorded in infants of women in the induction‐of‐labour group (1.4%) and one in the expectant‐management group (0.5%). Two cases of shoulder dystocia were resolved by the application of suprapubic pressure and the McRoberts manoeuvre. In the remaining two cases, internal manoeuvres were required. The allocation to induction of labour versus expectant management group for the actions required to relieve shoulder dystocia, is not reported.
Overall completeness and applicability of evidence
Only one trial was included in this review. Therefore, it was not possible to conduct a meta‐analysis of data for any outcome and there is insufficient evidence to assess the effect of planned birth, at or near term (37 to 40 weeks' gestation) for women with gestational diabetes. The trial gives an indication of the range of outcomes that have been measured to date.
The incidence of caesarean section in Alberico 2017 (12.6% among women in the induction‐of‐labour group and 11.8% in women in the expectant‐management group) is generally lower when compared to reported rates in the contemporary literature. The authors of the study suggest that this might be because of their criteria to exclude women experiencing major risk factors for caesarean section (e.g. maternal disease, previous caesarean section, fetal distress, estimated fetal weight of more than 4000 g).
Although Alberico 2017 is the only trial eligible for inclusion in this review, it is underpowered, in spite of recruitment occurring over a four‐year period at eight different centres in three different countries. One reason suggested for this is the difficulty associated with the randomisation of women to a planned birth group rather than women making an informed choice of planned or expectant management. This should be considered in future research. The lack of data on optimal interventions in relation to planned birth for this population of women, the existence of guidelines informed by retrospective studies, and the lack of clinical consensus on the most appropriate care option (Maso 2011) highlight the importance of research focused on women with gestational diabetes.
Quality of the evidence
This review included only one trial with 425 women with gestational diabetes.
Overall, the risk of bias in the study was assessed as low (if outcomes other than intact perineum are grouped for assessment of attrition bias for maternal outcomes).
We used the GRADE approach to assess the quality of evidence. All outcomes included in our Table 1, were downgraded for lack of blinding because it was felt that knowledge of allocation could potentially affect the outcomes. In addition, all outcomes were downgraded by two levels for imprecision, other than large‐for‐gestational age which was downgraded by one level. Of the seven outcomes included in our Table 1, maternal mortality or serious maternal morbidity, caesarean section, instrumental vaginal birth, perinatal mortality and shoulder dystocia were all graded as very low‐quality evidence. Large‐for‐gestational age was graded as low‐quality evidence and there were no data available for acidaemia.
The Institute for Maternal and Child Health, IRCCS, Burlo Garofolo offered “human and financial resources to carry out the project” (Alberico 2017). The authors of the Alberico 2017 study declared no conflict of interest.
Potential biases in the review process
This review was conducted in line with the guidance and procedures outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011), therefore minimising the introduction of bias during the review process. We undertook a comprehensive systematic search of databases and so are confident that all relevant studies were identified. We attempted to minimise bias by ensuring two review authors (LB and DD) independently assessed all potential studies identified by the search for inclusion, judged risk of bias, and extracted data.
Agreements and disagreements with other studies or reviews
Our findings demonstrate that there is limited high‐quality evidence to inform decisions on the effectiveness of planned birth at or near term gestation for women with gestational diabetes compared with an expectant approach. This was also the case in the conclusion of another review (Witkop 2009). The Witkop 2009 review included one randomised trial and four observational studies. While the findings of the Witkop 2009 review noted that the included observational studies suggest a potential reduction in macrosomia and shoulder dystocia with induction of labour and planned caesarean birth, the trial included in the review (Henry 1992), demonstrated no clear difference in rates of caesarean birth, shoulder dystocia, neonatal hypoglycaemia or perinatal deaths in the outcomes for women in the induction of labour versus the women in the expectant management group (Witkop 2009). It should be noted that published data from the trial included in the Witkop 2009 review does not differentiate between pre‐existing and gestational diabetes and therefore has been allocated to the studies awaiting classification for this review (See Characteristics of studies awaiting classification).
Authors' conclusions
Implications for practice.
There is limited evidence to inform implications for practice. Data that are available are not of high quality and lack power to detect possible important differences in either benefit or harm.
Implications for research.
There is an urgent need for high‐quality trials evaluating the effectiveness of planned birth at or near term gestation for women with gestational diabetes compared with an expectant approach. Given the equipoise on optimal approach, researchers planning these trials could consider strategies to optimise recruitment of women to their studies. This could include strategies to explain clearly the concept of equipoise with specific reference to the proposed study.
Notes
The original review 'Elective delivery in diabetic pregnant women' (Boulvain 2001) has now been split into two reviews.
Planned birth at or near term for improving health outcomes for pregnant women with gestational diabetes and their infants (this review).
Planned birth at or near term for improving health outcomes for pregnant women with pre‐existing diabetes and their infants (Biesty 2017a).
Acknowledgements
Our thanks to Dr Luca Ronfani who provided additional data for Alberico 2017.
As part of the pre‐publication editorial process, this review has been commented on by three peers (an editor and two referees who are external to the editorial team), a member of Cochrane Pregnancy and Childbirth's international panel of consumers and the group's Statistical Adviser.
This project was supported by the National Institute for Health Research, via Cochrane Programme Grant funding to Cochrane Pregnancy and Childbirth. The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, NHS or the Department of Health.
Appendices
Appendix 1. Search terms for ICTRP and ClinicalTrials.gov
ClinicalTrials.gov and the WHO International Clinical Trials Registry Platform.
WHO International Clinical Trials Registry Platform (ICTRP)
(we ran each line and alternative spelling separately)
GDM AND c(a)esarean
diabetes AND c(a)esarean
diabetic AND c(a)esarean
planned AND birth AND GDM
planned AND birth AND diabetes
planned AND birth AND diabetic
elective AND birth AND GDM
elective AND birth AND diabetes
elective AND birth AND diabetic
induction AND labo(u)r AND diabetes
induction AND labo(u)r AND GDM
Induction AND labo(u)r AND diabetic
expectant AND birth AND GDM
expectant AND birth AND diabetic
expectant AND birth AND diabetes
ClinicalTrials.gov
(we ran each search separately)
Advanced search
1.
diabetes OR diabetic OR GDM ‐ Condition
cesarean OR caesarean – Intervention
2.
diabetic OR diabetes OR GDM ‐ Condition
(planned OR elective OR expectant) AND (birth OR delivery) ‐ Intervention
3.
diabetes OR diabetic OR GDM ‐ Condition
induction AND (labour OR labor)
Data and analyses
Comparison 1. Planned elective birth (induction or caesarean) versus expectant.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Maternal mortality or serious maternal morbidity | 1 | 425 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.48 [0.25, 8.76] |
| 2 Caesarean section | 1 | 425 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.06 [0.64, 1.77] |
| 3 Instrumental vaginal birth | 1 | 425 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.81 [0.45, 1.46] |
| 4 Perinatal mortality rate | 1 | 425 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 5 Shoulder dystocia | 1 | 425 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.96 [0.31, 28.21] |
| 6 Large‐for‐gestational age | 1 | 425 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.53 [0.28, 1.02] |
| 7 Maternal death | 1 | 425 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 8 Admission to ICU | 1 | 425 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.48 [0.25, 8.76] |
| 9 Intact perineum | 1 | 373 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.02 [0.73, 1.43] |
| 10 Uterine rupture | 1 | 425 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 11 Postpartum haemorrhage | 1 | 425 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.17 [0.53, 2.54] |
| 12 Brachial plexus injury | 1 | 425 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 13 Bone fracture at birth | 1 | 425 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 14 Respiratory distress syndrome | 1 | 425 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.48 [0.25, 8.76] |
| 15 Neonatal hypoglycaemia | 1 | 425 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.74 [0.26, 2.09] |
| 16 Neonatal hyperbilirubinaemia | 1 | 397 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.46 [1.11, 5.46] |
| 17 Admission to NICU | 1 | 425 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.99 [0.14, 6.94] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Alberico 2017.
| Methods |
Study design: multicentre open‐labelled randomised controlled trial. Duration of study: 4 years (recruitment phase). Study dates: March 2010 ‐ March 2014 (recruitment phase). |
|
| Participants |
Setting: 8 'centres' in 3 countries (Italy, Slovenia, Israel). Inclusion criteria: pregnant women, older than 18, with singleton pregnancy, vertex presentation and diagnoses of gestational diabetes in this pregnancy. Exclusion criteria: diagnosis of overt diabetes, prior caesarean section, obstetrical contraindications to vaginal delivery, maternal pregnancy‐related disease or non‐reassuring fetal well‐being necessitating prompt delivery, uncertain gestational age, known fetal anomaly, Bishop score > 7, or estimated fetal weight of 4000 g at enrolment. Participants randomised (#): 425 women (530 women were recruited, 59 women opted out and 46 women were not eligible). Characteristics for planned subgroup analysis: Parity: mixed. Both nulliparous and multiparous women included (% presented in Table 1 of manuscript). 125/214 (59.4%) nulliparous women in planned birth group versus 104/211 (49.3%) nulliparous women in expectant approach group. 87/214 (40.6%) multiparous women in planned group versus 107/211 (50.7%) multiparous women in the expectant approach group. |
|
| Interventions |
Planned birth: N = 214 women. Between 38‐39 weeks of gestation, women in this group were admitted to the ward and induction of labour was performed. "IOL will be performed using dinoprostone, 2 mg vaginally, or dinoprostone, 0.5 mg intracervically in 6‐8 hours intervals (up to 5 doses) or dinoprostone 10 mg vaginal device. Once the patient’s Bishop score exceeds 7 or regular contractions are diagnosed, patients will be transferred to the delivery ward for artificial rupture of membranes or oxytocin augmentation as indicated. Patients, in which cervical ripening does not occur (Bishops score < 7) after 5 attempts with prostaglandin E2 (PGE2), will be offered either oxytocin or Foley catheter induction or caesarean section, according to protocol" (Published Protocol, Maso et al 2011:4). 2 women in this group (0.9%) presented with spontaneous onset of labour. Expectant approach: N = 211 women. Women enrolled in this group (between 38‐39 weeks' gestation) were followed up twice weekly by electronic fetal heart rate monitoring and biophysical profile until 41 weeks' gestation, when induction of labour was then offered. "...patients enrolled in the conservative management arm will be followed up twice weekly by Electronic Fetal Heart Rate monitoring and Biophysical Profile. Patients will be followed up until 41 weeks' gestation. Women, who do not deliver by this gestational age, will be admitted for IOL. IOL will be offered when non‐reassuring fetal status is suspected. All patients in the conservative arm will undergo fetal weight ultrasound estimation prior to IOL. Patients with estimated fetal weight of over 4000 g will be offered a caesarean section" (Published Protocol, Maso et al 2011:4). 50 women in this group (23.7%) underwent induction of labour for "obstetric or medical indications". |
|
| Outcomes | Outcomes considered in the review and reported in or extracted from the study: outcome data for serious maternal morbidity, caesarean section, instrumental birth, intact perineum, postpartum haemorrhage, admission to ICU, perinatal mortality rate, shoulder dystocia, large‐for‐gestational age, birth trauma, respiratory distress syndrome, neonatal hypoglycaemia, neonatal hyperbilirubinaemia, admission to NICU. | |
| Notes |
Funding: The Institute for Maternal & Child Health, IRCCS, Burlo Garofolo "offered human and financial resources to carry out the project". Trial authors’ declaration of interest: none declared. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Randomisation was centralised and coordinated by the Clinical Epidemiology and Biostatistics Unit (IRCCS) using a computer based method. The randomisation list was blocked and stratified by centre." |
| Allocation concealment (selection bias) | Low risk | "The allocation concealment was guaranteed through the use of consecutively numbered and sealed opaque envelopes." |
| Blinding of participants and personnel (performance bias) All outcomes | High risk |
Participants: “The study was an open‐label trial because of the practical impossibility to blind either healthcare professionals or patients to the allocation group“. Personnel: “The study was an open‐label trial because of the practical impossibility to blind either healthcare professionals or patients to the allocation group“. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Not stated explicitly but unlikely given following extract from study report. “General information on recruited patients, outcomes and randomization group will be indicated in an appropriate paper questionnaire in the first instance and then reported on an electronic database accessible from different study sites in the second instance. In each participating centre, medical personnel will fill up the electronic form directly.“ |
| Incomplete outcome data (attrition bias) Maternal‐perineum intact | High risk | Outcome data available for all women randomised (induction 214, expectant 211) other than perineal trauma where data are available for 198/211, 94% (induction) and 175/214, 82% (expectant). |
| Incomplete outcome data (attrition bias) Maternal‐all outcomes (other than intact perineum) | Low risk | Outcome data available for all women randomised (induction 214, expectant 211) other than perineal trauma where data are available for 198/211, 94% (induction) and 175/214, 82% (expectant). |
| Incomplete outcome data (attrition bias) Neonatal‐all outcomes | Low risk | Outcome data available for all infants of mothers randomised with exception of hyperbilirubinaemia, which is available for 93.45% of babies of women in the induction of labour group and 92% in the expectant management group. |
| Selective reporting (reporting bias) | Low risk | Published and registered protocol available. All outcomes stated in protocol other than 10‐minute Apgar score and uterine rupture reported in paper. |
| Other bias | Low risk | No other obvious risk of bias identified. |
ICU: intensive care unit IOL: induction of labour NICU: neonatal intensive care unit
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Ghosh 1979 | Focus: "Type B diabetes". No further details or publications in the intervening period (38 years). |
| Khojandi 1974 | Insufficient information to determine randomisation. Given that this study was published 42 years ago, we did not attempt to contact the authors. |
| Worda 2017 | This trial randomised women to either induction of labour at 38 weeks' gestation or 40 weeks' gestation — these intervention groups were not compared with an expectation approach. |
Characteristics of studies awaiting assessment [ordered by study ID]
Dhaneshwor 2011 (CTRI/2011/12/002290).
| Methods |
Study design: "Randomised, parallel group, active controlled trial". Duration of the study: protocol registered (CTRI/2011/12/002290) on 26/12/2011. |
| Participants |
Setting: Vellore, Tamil Nadu. Inclusion criteria: "Maternal age ? 18 years, willing for delivery at Christian Medical College (CMC) Vellore, singleton pregnancy in vertex presentation, gestational age between 39+ and 40 weeks, by LMP sure of dates or by early scan." Exclusion criteria: "Women not willing for delivery at CMC, Vellore, Prior C‐section, suspected estimated fetal weight of > 3.5 kg or any known contraindications to vaginal delivery, uncertain gestational age, non reassuring fetal wellbeing necessitating delivery, maternal pregnancy‐related disease necessitating delivery, known fetal anomaly". |
| Interventions |
Planned birth: "Induction group ‐ for those in the induction group arm, cervical ripening will be done with Misoprostol (25mcg every 6th hourly [sic] for 2 doses) as is the routine for induction of labour in our hospital. A 3rd dose will be used if the cervix is still unfavourable and NST is reactive. If cervix remains unfavourable after 3 doses, patient may be re‐induced after 2‐3 days as situation warrants or as per the parent treating unit protocol (PGE1 25mcg Q6h 2 dose)". Expectant approach: "Expectant Group ‐ for those allocated to expectant group, they will be followed up until delivery, with AFI (amniotic fluid index) and NST (non stress test) twice a week. In absence of spontaneous delivery, labour will be induced at 40+6 weeks. In presence of non‐reassuring fetal well‐being at one of the follow‐ups, she will immediately be offered labour induction". |
| Outcomes | Outcomes considered relevant to this review: at time of birth: caesarean section, model of delivery, presence of perineal tears, postpartum haemorrhage, shoulder dystocia, newborn birthweight, arterial cord pH. At time of discharge: maternal or neonatal intensive care, maternal and perinatal death, neonatal birth trauma, neonatal respiratory distress, neonatal hyperbilirubinaemia, neonatal hypoglycaemia. |
| Notes |
Funding: not known. Trial authors' declaration of interest: none declared. The authors were contacted on: 10th Jan 2017 (to obtain more information in relation to the study, including the status of the study, findings and potential publications). |
Henry 1992.
| Methods |
Study design: "Randomised Trial". Duration of the study: 3.5 years. |
| Participants |
Setting: Women's Hospital, Los Angeles County — University of Southern California Medical Center. Inclusion criteria: "women diagnosed before pregnancy with insulin‐dependent diabetes mellitus or non‐insulin‐dependent diabetes mellitus without vascular complications or with gestational diabetes requiring insulin treatment during pregnancy, and with good metabolic control of blood glucose levels ... 38 completed weeks' gestation (266 days), good compliance with clinical appointments and home blood glucose monitoring, no abnormalities in the twice weekly antepartum assessment with nonstress testing and amniotic fluid volume measurement performed from 34 weeks onward, singleton gestation and cephalic presentation, clinical and ultrasonographic estimation of fetal weight <3800gm at 38 completed weeks with no evidence of intrauterine growth retardation, no other medical or obstetric complications, a candidate for trial of vaginal delivery (no more than 2 previous C‐sections)". |
| Interventions |
Planned birth: N = 100 women, "labour was induced with iv oxytocin .... in women with favourable Bishops Score (<4), unscarred uteri and normal amniotic fluid indexes (>5.0cms), up to 3 applications of vaginal prostaglandin (3 mg) were used for cervical ripening before oxytocin treatment". Expectant approach: N = 100 women, "expectant management consisted of daily split dose insulin therapy and home blood glucose monitoring, weekly antenatal clinic visits, and twice‐weekly antepartum testing". Induction of labour indicated by: "suspected fetal distress .... preeclampsia, maternal hyperglycaemia, estimated fetal weight > 4200gm, 42 weeks gestation". |
| Outcomes | Outcomes considered relevant to this review: mode of delivery — vaginal delivery, caesarean delivery; infant birthweight — macrosomia, large‐for‐gestational age; infant outcome — shoulder dystocia, birth trauma (bone fracture, Erbs palsy), hypoglycaemia, mortality. |
| Notes |
Funding: not known. Trial authors declaration of Interest: none declared. The authors were contacted on: 10th Jan 2017 (to obtain data specifically related to women with gestational diabetes in the planned birth versus expectant approach groups). |
Differences between protocol and review
The title has changed from 'Planned elective birth at or near term for improving health outcomes for pregnant women with gestational diabetes', to 'Planned birth at or near term for improving health outcomes for pregnant women with gestational diabetes and their infants'.
The protocol for this Cochrane review was published in PROSPERO on 16 August 2017 — see http://www.crd.york.ac.uk/PROSPERO/display_record.asp?ID=CRD42017072476. The protocol was not published in theCochrane Library.
Contributions of authors
Aoife Egan (AE) and Fidelma Dunne (FD) drafted the background section. All other authors contributed to editing the background text. All authors contributed to the drafting of the inclusion criteria for the review. Linda Biesty (LB) and Declan Devane (DD) drafted the methodology and all authors read and commented on this. LB and DD abstracted data including risk of bias. LB wrote the results section, discussion and implications sections with input from all authors. LB is the guarantor of this review.
Sources of support
Internal sources
No sources of support supplied
External sources
National Institute for Health Research (NIHR), NIHR Cochrane Programme Grant Project: 13/89/05 – Pregnancy and childbirth systematic reviews to support clinical guidelines, UK.
Declarations of interest
Linda M Biesty: none known.
Aoife M Egan: none known.
Fidelma Dunne: none known.
Eugene Dempsey: none known.
Pauline Meskell: none known.
Valerie Smith: none known.
Méabh Ní Bhuinneáin: none known.
Declan Devane: none known.
New
References
References to studies included in this review
Alberico 2017 {published data only}
- Alberico S, Erenbourg A, Hod M, Yogev Y, Hadar E, Neri F, et al. Immediate delivery or expectant management in gestational diabetes at term: the ginexmal randomised controlled trial. BJOG: an international journal of obstetrics and gynaecology 2017;124(4):669‐77. [DOI] [PubMed] [Google Scholar]
- Maso G, Alberico S, Wiesenfeld U, Ronfani L, Erenbourg A, Hadar E, et al. GINEXMAL RCT: induction of labour versus expectant management in gestational diabetes pregnancies. BMC Pregnancy and Childbirth 2011;11:31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NCT01058772. GINEXMAL RCT: Induction of labour versus expectant management in gestational diabetes pregnancies. clinicaltrials.gov/ct2/show/NCT01058772 (first received: 26 January 2010).
References to studies excluded from this review
Ghosh 1979 {published data only}
- Ghosh S, Khakoo H, Pillari VT, Carmona RA, Rajegoda BK, Poliak A. Timing of delivery in rigidly controlled class B diabetes [abstract]. 9th World Congress of Gynecology and Obstetrics; 1979 October 26‐31; Tokyo, Japan. 1979:270‐1.
Khojandi 1974 {published data only}
- Khojandi M, Tsai AY/M, Tyson JE. Gestational diabetes: the dilemma of delivery. Obstetrics & Gynecology 1974;43:1‐6. [PubMed] [Google Scholar]
Worda 2017 {published data only}
- Husslein P, NCT01256892. Insulin dependent gestational diabetes mellitus: randomized trial of induction of labour at 38 and 40 weeks of gestation. clinicaltrials.gov/ct2/show/NCT01256892 (first received 7 December 2010).
- Worda K, Bancher‐Todesca D, Husslein P, Worda C, Leipold H. Randomized controlled trial of induction at 38 weeks versus 40 weeks gestation on maternal and infant outcomes in women with insulin‐controlled gestational diabetes. Wiener Klinische Wochenschrift [epub ahead of print]. [DOI] [PubMed]
References to studies awaiting assessment
Dhaneshwor 2011 (CTRI/2011/12/002290) {published data only}
- CTRI/2011/12/002290. Gestational diabetes well controlled on medical nutritional therapy: a randomized trial of active induction of labour compared with expectant management. ctri.nic.in/Clinicaltrials/pmaindet2.php?trialid=3618 (first received 26 December 2011).
Henry 1992 {published data only}
- Henry OA, Kjos SL, Montoro M, Buchanan TA, Mestman JH. Randomized trial of elective induction vs expectant management in diabetics. American Journal of Obstetrics and Gynecology 1992;166:304. [DOI] [PubMed] [Google Scholar]
- Kjos SL, Henry OA, Montoro M, Buchanan TA, Mestman JH. Insulin‐requiring diabetes in pregnancy: a randomized trial of active induction of labour and expectant management. American Journal of Obstetrics and Gynecology 1993;169:611‐5. [DOI] [PubMed] [Google Scholar]
Additional references
ACOG 2013
- American College of Obstetricians and Gynecologists Committee on Practice Bulletins ‐ Obstetrics. ACOG Practice Bulletin. Clinical management guidelines for obstetrician‐gynecologists. Number 137, August 2013 (replaces Practice Bulletin Number 30, September 2001). Gestational diabetes mellitus. Obstetrics and Gynecology 2013;122(2 Pt 1):406‐16. [PubMed] [Google Scholar]
ACOG 2017
- American College of Obstetricians and Gynecologists Committee on Practice Bulletins ‐ Obstetrics. ACOG Practice Bulletin. Clinical management guidelines for obstetrician‐gynecologists. Number 180, July 2017 Gestational Diabetes Mellitus. Obstetrics and Gynecology 2017;130(1):e17‐e37. [DOI] [PubMed] [Google Scholar]
ADA 2017
- American Diabetes Association. Standards of medical care in diabetes. Diabetes Care 2017;40(Supp 1):S11‐S24. [Google Scholar]
Aktun 2015
- Aktun LH, Yorgunlar B, Karaca N, Akpak YK. Predictive risk factors in the treatment of gestational diabetes mellitus. Clinical Medicine Insights. Womens Health 2015;14(8):25‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Avalos 2013
- Avalos GE, Owens LA, Dunne F (ATLANTIC DIP Collaborators). Applying current screening tools for gestational diabetes mellitus to a European population: is it time for change?. Diabetes Care 2013;36(10):3040‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Benhalima 2015
- Benhalima K, Mathieu C, Damm P, Assche A, Devlieger R, Desoye G, et al. A proposal for the use of uniform diagnostic criteria for gestational diabetes in Europe: an opinion paper by the European Board & College of Obstetrics and Gynaecology (EBCOG). Diabetologia 2015;58(7):1422‐9. [DOI] [PubMed] [Google Scholar]
Benhalima 2016
- Benhalima K, Mathieu C, Assche A, Damm P, Devlieger R, Mahmood T, et al. Survey by the European Board and College of Obstetrics and Gynaecology on screening for gestational diabetes in Europe. European Journal of Obstetrics, Gynecology, and Reproductive Biology 2016;201:197‐202. [DOI] [PubMed] [Google Scholar]
Biesty 2017a
- Biesty LM, Egan AM, Dunne F, Smith V, Meskell P, Dempsey E, et al. Planned birth at or near term for improving health outcomes for pregnant women with pre‐existing diabetes and their infants. Cochrane Database of Systematic Reviews (in press). [DOI] [PMC free article] [PubMed] [Google Scholar]
Bilous 2015
- Bilous R. Diagnosis of gestational diabetes, defining the net, refining the catch. Diabetologia 2015;58(9):1965‐8. [DOI] [PubMed] [Google Scholar]
Blumer 2013
- Blumer I, Hadar E, Hadden DR, Jovanovič L, Mestman JH, Murad MH. Diabetes and pregnancy: an endocrine society clinical practice guideline. Journal of Clinical Endocrinology and Metabolism 2013;98(11):4227‐49. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bond 2017
- Bond DM, Middleton P, Levett KM, Ham DP, Crowther CA, Buchanan SL, et al. Planned early birth versus expectant management for women with preterm prelabour rupture of membranes prior to 37 weeks' gestation for improving pregnancy outcome. Cochrane Database of Systematic Reviews 2017, Issue 3. [DOI: 10.1002/14651858.CD004735.pub4] [DOI] [PMC free article] [PubMed] [Google Scholar]
Bottalico 2007
- Bottalico JN. Recurrent gestational diabetes: risk factors, diagnosis, management, and implications. Seminars in Perinatology 2007;31(3):176‐84. [DOI] [PubMed] [Google Scholar]
Boulvain 2016
- Boulvain M, Irion O, Dowswell T, Thornton JG. Induction of labour at or near term for suspected fetal macrosomia. Cochrane Database of Systematic Reviews 2016, Issue 5. [DOI: 10.1002/14651858.CD000938.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Brown 2016
- Brown J, Grzeskowiak L, Williamson K, Downie MR, Crowther CA. Insulin for the treatment of women with gestational diabetes. Cochrane Database of Systematic Reviews 2016, Issue 1. [DOI: 10.1002/14651858.CD012037] [DOI] [PMC free article] [PubMed] [Google Scholar]
Brown 2017a
- Brown J, Martis R, Hughes B, Rowan J, Crowther CA. Oral anti‐diabetic pharmacological therapies for the treatment of women with gestational diabetes. Cochrane Database of Systematic Reviews 2017, Issue 1. [DOI: 10.1002/14651858.CD011967.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Brown 2017b
- Brown J, Alwan NA, West J, Brown S, McKinlay CJD, Farrar D, et al. Lifestyle interventions for the treatment of women with gestational diabetes. Cochrane Database of Systematic Reviews 2017, Issue 5. [DOI: 10.1002/14651858.CD011970.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Brudenell 1989
- Brudenell M, Doddridge M. Delivering the infant. In: Lind T editor(s). Current Reviews in Obstetrics and Gynecology. Diabetic Pregnancy. Vol. 13, New York: Churchill Livingstone, 1989. [Google Scholar]
Buckley 2012
- Buckley BS, Harreiter J, Damm P, Corcoy R, Chico A, Simmons D, et al. Gestational diabetes mellitus in Europe: prevalence, current screening practice and barriers to screening. A review. Diabetic Medecine 2012;29(7):844‐54. [DOI] [PubMed] [Google Scholar]
Carolan 2012
- Carolan M, Davey MA, Biro MA, Kealey M. Maternal age, ethnicity and gestational diabetes mellitus. Midwifery 2012;28(6):778‐83. [DOI] [PubMed] [Google Scholar]
Catalano 2011
- Catalano PM, Hauguel‐de Mouzon S. Is it time to revisit the Pedersen hypothesis in the face of the obesity epidemic?. American Journal of Obstetrics and Gynecology 2011;204(6):479‐87. [DOI] [PMC free article] [PubMed] [Google Scholar]
Chamberlain 2013
- Chamberlain C, McNamara B, Williams E, Yore D, Oldenburg B, Oats J, et al. Diabetes in pregnancy among indigenous women in Australia, Canada, New Zealand and the United States. Diabetes Metabolism Research Review 2013;29(4):241‐56. [DOI] [PMC free article] [PubMed] [Google Scholar]
Chen 2015
- Chen P, Wang S, Ji J, Ge A, Chen C, Zhu Y, et al. Risk factors and management of gestational diabetes. Cell Biochemistry and Biophysics 2015;71(2):689‐94. [DOI] [PubMed] [Google Scholar]
Crowther 2005
- Crowther CA, Hiller JE, Ross JR, McPhee AJ, Jefferies WS, Robinson JS. Effect of treatment of gestational diabetes mellitus on pregnancy outcomes. New England Journal of Medicine 2005;352(24):2477‐86. [DOI] [PubMed] [Google Scholar]
Dodd 2014
- Dodd JM, Deussen AR, Grivell RM, Crowther CA. Elective birth at 37 weeks' gestation for women with an uncomplicated twin pregnancy. Cochrane Database of Systematic Reviews 2014, Issue 2. [DOI: 10.1002/14651858.CD003582.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Duman 2015
- Duman NB. Frequency of gestational diabetes mellitus and the associated risk factors. Pakistan Journal of Medical Sciences Quarterly 2015;3(1):194‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
EPOC 2016
- Effective Practice, Organisation of Care (EPOC). What study designs should be included in an EPOC review? EPOC Resources for review authors. Oslo: Norwegian Knowledge Centre for the Health Services 2016; Vol. Available at: http://epoc.cochrane.org/epoc‐specific‐resources‐review‐authors.
Farrar 2016
- Farrar D, Tuffnell DJ, West J, West HM. Continuous subcutaneous insulin infusion versus multiple daily injections of insulin for pregnant women with diabetes. Cochrane Database of Systematic Reviews 2016, Issue 6. [DOI: 10.1002/14651858.CD005542.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Farrar 2017
- Farrar D, Duley L, Dowswell T, Lawlor DA. Different strategies for diagnosing gestational diabetes to improve maternal and infant health. Cochrane Database of Systematic Reviews 2017, Issue 8. [DOI: 10.1002/14651858.CD007122.pub4] [DOI] [PMC free article] [PubMed] [Google Scholar]
Feig 2015
- Feig DS, Corcoy R, Jensen DM, Kautzky‐Willer A, Nolan CJ, Oats JJ, et al. Diabetes in pregnancy outcomes: a systematic review and proposed codification of definitions. Diabetes Metabolism Research and Reviews 2015;31(7):680‐90. [DOI] [PubMed] [Google Scholar]
Gulmezoglu 2012
- Gülmezoglu AM, Crowther CA, Middleton P, Heatley E. Induction of labour for improving birth outcomes for women at or beyond term. Cochrane Database of Systematic Reviews 2012, Issue 6. [DOI: 10.1002/14651858.CD004945.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Hod 2015
- Hod M, Kapur A, Sacks DA, Hadar E, Agarwal M, Renzo GC, et al. The International Federation of Gynecology and Obstetrics (FIGO) Initiative on gestational diabetes mellitus: A pragmatic guide for diagnosis, management, and care. International Journal of Gynaecology and Obstetrics 2015;131(Suppl 3):S173‐S211. [DOI] [PubMed] [Google Scholar]
IADPSG Consensus Panel 2010
- International Association of Diabetes and Pregnancy Study Groups. Recommendations on the diagnosis and classification of hyperglycemia in pregnancy. International Association of Diabetes and Pregnancy Study Groups Consensus Panel. Diabetes Care 2010;33(3):676‐82. [DOI] [PMC free article] [PubMed] [Google Scholar]
Irion 1998
- Irion O, Boulvain M. Induction of labour for suspected fetal macrosomia. Cochrane Database of Systematic Reviews 1998, Issue 2. [DOI: 10.1002/14651858.CD000938] [DOI] [PubMed] [Google Scholar]
Jafari‐Shobeiri 2015
- Jafari‐Shobeiri M, Ghojazadeh M, Azami‐Aghdash S, Naghavi‐Behzad M, Piri R, Pourali‐Akbar Y, et al. Prevalence and risk factors of gestational diabetes in Iran: A systematic review and meta‐analysis. Iranian Journal of Public Health 2015;44(8):1036‐44. [PMC free article] [PubMed] [Google Scholar]
Ju 2009
- Ju H, Chadha Y, Donovan T, O'Rourke P. Fetal macrosomia and pregnancy outcomes. Australian & New Zealand Journal of Obstetrics & Gynaecology 2009;49(5):504‐9. [DOI] [PubMed] [Google Scholar]
Kgosidialwa 2015
- Kgosidialwa O, Egan AM, Carmody L, Kirwan B, Gunning P, Dunne FP. Treatment with diet and exercise for women with gestational diabetes mellitus diagnosed using IADPSG criteria. Journal of Clinical Endocrinology and Metabolism 2015;100(12):4629‐36. [DOI] [PubMed] [Google Scholar]
Khireddine 2013
- Khireddine I, Ray C, Dupont C, Rudigoz RC, Bouvier‐Colle MH, Deneux‐Tharaux C. Induction of labor and risk of postpartum hemorrhage in low risk parturients. PLOS One 2013;8(1):e54858. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kim 2002
- Kim C, Newton KM, Knopp RH. Gestational diabetes and the incidence of type 2 diabetes: a systematic review. Diabetes Care 2002;25(10):1862‐8. [DOI] [PubMed] [Google Scholar]
Kim 2013
- Kim SY, Saraiva C, Curtis M, Wilson HG, Troyan J, Sharma AJ. Fraction of gestational diabetes mellitus attributable to overweight and obesity by race/ethnicity, California 2007‐2009. American Journal of Public Health 2013;103(10):e65‐72. [DOI] [PMC free article] [PubMed] [Google Scholar]
Krakowiak 2012
- Krakowiak P, Walker CK, Bremer AA, Baker AS, Ozonoff S, Hansen RL, et al. Maternal metabolic conditions and risk for autism and other neurological conditions. Pediatrics 2012;129(5):e1121‐1128. [DOI] [PMC free article] [PubMed] [Google Scholar]
Macer 1992
- Macer JA, Macer CL, Chan LS. Elective induction versus spontaneous labor: a retrospective study of complications and outcome. American Journal of Obstetrics and Gynecology 1992;166:1690‐7. [DOI] [PubMed] [Google Scholar]
Maso 2011
- Maso G, Alberico S, Wiesenfeld U, Ronfani L, Erenbourg A, Hadar E, et al. GINEXMAL RCT: induction of labour versus expectant management in gestational diabetes pregnancies. BMC Pregnancy and Childbirth 2011;11:31. [DOI] [PMC free article] [PubMed] [Google Scholar]
McCurdy 2010
- McCurdy C, Friedman J. Mechanisms underlying insulin resistance in human pregnancy and gestational diabetes mellitus. In: Kim K, Ferrara A editor(s). Gestational Diabetes During and After Pregnancy. London: Springer, 2010:125‐38. [Google Scholar]
McIntyre 2016
- McIntyre HD, Sacks DA, Barbour LA, Feig DS, Catalano PM, Damm P, e al. Issues with the diagnosis and classification of hyperglycemia in early pregnancy. Diabetes Care 2016;39(1):53‐4. [DOI] [PubMed] [Google Scholar]
Metzger 1985
- Metzger BE, Bybee DE, Freinkel N, Phelps RL, Radvany RM, Vaisrub N. Gestational diabetes mellitus. Correlations between the phenotypic and genotypic characteristics of the mother and abnormal glucose tolerance during the first year postpartum. Diabetes 1985;34(34 Suppl 2):111‐5. [DOI] [PubMed] [Google Scholar]
Metzger 2008
- Metzger BE, Lowe LP, Dyer AR, Trimble ER, Chaovarindr U, Coustan DR, et al. Hyperglycemia and adverse pregnancy outcomes. New England Journal of Medicine 2008;358(19):1991‐2002. [DOI] [PubMed] [Google Scholar]
Metzger 2010
- Metzger BE, Gabbe SG, Persson B, Buchanan TA, Catalano PA, Damm P, et al. International association of diabetes and pregnancy study groups recommendations on the diagnosis and classification of hyperglycemia in pregnancy. Diabetes Care 2010;33(3):676‐82. [DOI] [PMC free article] [PubMed] [Google Scholar]
NCT01058772
- NCT01058772. GINEXMAL RCT: Induction of labour versus expectant management in gestational diabetes pregnancies. clinicaltrials.gov/ct2/show/NCT01058772 (first received: 26th January 2010).
New Zealand Ministry of Health 2014
- New Zealand Ministry of Health. Screening, Diagnosis and Management of Gestational Diabetes in New Zealand: A Clinical Practice Guideline. Wellington, New Zealand: Ministry of Health 2014.
NICE 2015
- National Institute for Health and Clinical Excellence (NICE) and National Collaborating Centre for Women and Children's Health Project Team. Diabetes in pregnancy: Management of diabetes and its complications from pre‐conception to the postnatal period. NG3. NICE 2015.
Noctor 2016
- Noctor E, Crowe C, Carmody LA, Saunders, JA, Kirwan B, O’Dea A, et al (for the ATLANTIC DIP investigators). Cumulative incidence of abnormal glucose tolerance up to 5 years post‐gestational diabetes mellitus with use of Internation Association of Diabetes and Prenancy Study Group criteria. European Journal of Endocrinology 2016;175(4):287‐97. [DOI] [PubMed] [Google Scholar]
O’Dea 2015
- O'Dea A, Tierney M, McGuire BE, Newell J, Glynn LG, Gibson I, et al. Can the onset of type 2 diabetes be delayed by a group‐based lifestyle intervention in women with prediabetes following gestational diabetes mellitus (GDM)? Findings from a randomized control mixed methods trial. Journal of Diabetes Research 2015;2015:798460. [DOI: 10.1155/2015/798460] [DOI] [PMC free article] [PubMed] [Google Scholar]
O’Sullivan 1980
- O'Sullivan JB. Establishing criteria for gestational diabetes. Diabetes Care 1980;3(3):437‐9. [DOI] [PubMed] [Google Scholar]
O’Sullivan 2012
- O'Sullivan EP, Avalos G, O'Reilly M, Dennedy MC, Gaffney G, Dunne FP (Atlantic DIP collaborators). Atlantic DIP: the prevalence and consequences of gestational diabetes in Ireland. Irish Medical Journal 2012;105(5 Suppl):13‐5. [PubMed] [Google Scholar]
Page 2014
- Page KA, Romero A, Buchanan TA, Xiang AH. Gestational diabetes mellitus, maternal obesity and adiposity in offspring. Journal of Pediatrics 2014;164(4):807‐10. [DOI] [PMC free article] [PubMed] [Google Scholar]
Perlow 1996
- Perlow JH, Wigton T, Hart J, Strassner HT, Nageotte MP, Wolk BM. Birth trauma: a five‐year review of incidence and associated perinatal factors. Journal of Reproductive Medicine 1996;41:754‐60. [PubMed] [Google Scholar]
Ramos‐Roman 2011
- Ramos‐Roman MA. Prolactin and lactation as modifiers of diabetes risk in gestational diabetes. Hormone and Metabolic Research 2011;43:593‐600. [DOI] [PubMed] [Google Scholar]
Reece 2010
- Reece EA. The fetal and maternal consequences of gestational diabetes mellitus. Journal of Maternal‐Fetal and Neonatal Medicine 2010;23(3):199‐203. [DOI] [PubMed] [Google Scholar]
RevMan 2014 [Computer program]
- Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager 5 (RevMan 5). Version 5.3. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Rosenstein 2012
- Rosenstein MG, Cheng YW, Snowden JM, Nicholson JM, Caughey AB. Risk of stillbirth and infant death stratified by gestational age. Obstetrics and Gynecology 2012;120(1):76‐82. [DOI] [PMC free article] [PubMed] [Google Scholar]
Schneider 2012
- Schneider S, Bock C, Wetzel M, Maul H, Loerbroks A. The prevalence of gestational diabetes in advanced economies. Journal of Perinatal Medicine 2012;40(5):511. [DOI] [PubMed] [Google Scholar]
Setji 2005
- Setji T, Brown A, Feinglos M. Gestational diabetes mellitus. Clinical Diabetes 2005;23:17‐24. [Google Scholar]
St Vincent Declaration 1990
- World Health Organization (Europe) and International Diabetes Federation (Europe). Diabetes care and research in Europe: the Saint Vincent Declaration. Diabetic Medicine 1990;7(4):360. [PubMed] [Google Scholar]
Tieu 2008
- Tieu J, Crowther CA, Middleton P. Dietary advice in pregnancy for preventing gestational diabetes mellitus. Cochrane Database of Systematic Reviews 2008, Issue 2. [DOI: 10.1002/14651858.CD006674.pub2] [DOI] [PubMed] [Google Scholar]
Wendland 2012
- Wendland EM, Torloni MR, Falavigna M, Trujillo J, Dode MA, Campos MA, et al. Gestational diabetes and pregnancy outcomes‐‐a systematic review of the World Health Organization (WHO) and the International Association of Diabetes in Pregnancy Study Groups (IADPSG) diagnostic criteria. BMC Pregnancy Childbirth 2012;12:23. [DOI] [PMC free article] [PubMed] [Google Scholar]
WHO 2014
- World Health Organization. Diagnostic criteria and classification of hyperglycaemia first detected in pregnancy: a World Health Organization Guideline. Diabetes Research and Clinical Practice 2014;103(3):341‐63. [DOI] [PubMed] [Google Scholar]
Wilcox 2005
- Wilcox G. Insulin and insulin resistance. Clinical Biochemistry Review 2005;26(5):19‐39. [PMC free article] [PubMed] [Google Scholar]
Witkop 2009
- Witkop CT, Neale D, Wilson LM, Bass EB, Nicholson WK. Active compared with expectant delivery management in women with gestational diabetes: a systematic review. Obstetrics and Gynecology 2009;113(1):206‐17. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Biesty 2017b
- Biesty LM, Egan AM, Dunne F, Dempsey E, Meskell P, Smith V, et al. Planned elective birth at or near term for improving health outcomes for pregnant women with gestational diabetes. PROSPERO 2017:CRD42017072476 Available from http://www.crd.york.ac.uk/PROSPERO/display_record.asp?ID=CRD42017072476. [DOI] [PMC free article] [PubMed]
Boulvain 2001
- Boulvain M, Stan CM, Irion O. Elective delivery in diabetic pregnant women. Cochrane Database of Systematic Reviews 2001, Issue 2. [DOI: 10.1002/14651858.CD001997] [DOI] [PMC free article] [PubMed] [Google Scholar]


