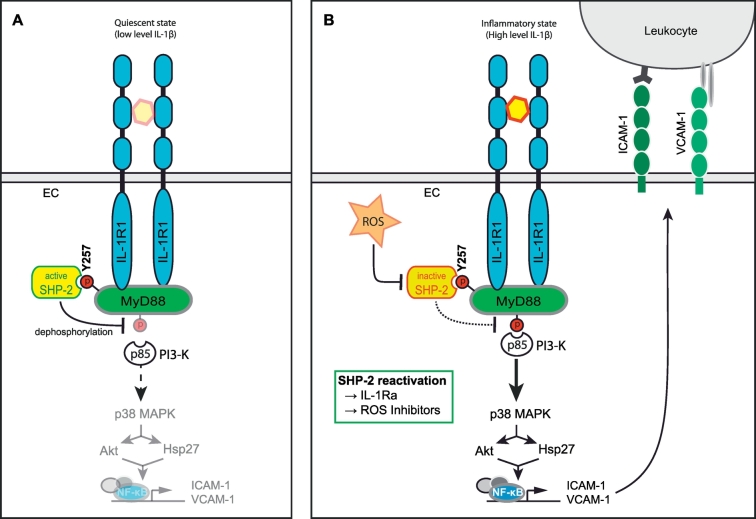
Fig. 6.
Proposed model for the role of SHP-2 in the regulation of IL-1β-dependent EC signalling and vascular inflammation. A) The tyrosine phosphatase SHP-2 possesses a basal activity under physiological conditions. Thus, SHP-2 may preserve endothelial quiescence by keeping MyD88 phospho-tyrosine sites in a dephosphorylated state, thereby limiting pro-inflammatory downstream signalling via interaction partners of MyD88 such as PI3-K. B) Upon activation of the IL-1R1 by IL-1β, MyD88 is phosphorylated on Y257 enabling binding of SHP-2. However, inflammation-induced generation of ROS results in inactivation of SHP-2 by oxidation of the critical cysteine residue in the phosphatase domain suspending the protective effect of SHP-2. Hence, a different SH-2 binding site is phosphorylated, resulting in recruitment of p85/PI3-K to MyD88 and subsequent activation of inflammatory downstream signalling including p38 MAPK, AKT, Hsp27 and NF-κB. Consequently, expression of ICAM-1 and VCAM-1 at the endothelial surface is induced followed by leukocyte recruitment as well as enhanced vascular leakage (not shown). The reactivation of SHP-2 activity by IL-1Ra or ROS inhibition may be a promising therapeutic approach to prevent the extensive inflammatory responses mediated by MyD88 during inflammatory diseases such as sepsis.