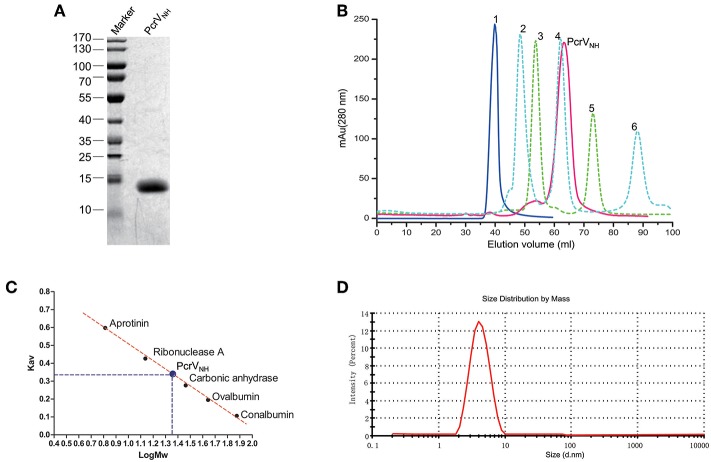
Figure 2.
Characterization of recombinant PcrVNH. (A) SDS-PAGE analysis of PcrVNH. The purity of PcrVNH was ~96% as determined by the density of the corresponding band on a SDS-PAGE gel. (B) The chromatograms curves of protein standards and PcrVNH were overlaid for comparison. Curves 1 to 6 represent the peaks of standard proteins, namely, blue dextran 2000, conalbumin, ovalbumin, carbonic anhydrase, ribonuclease A, and aprotinin. The corresponding elution volume was 40.0, 47.3, 54.6, 62.1, 73.8, and 88.2 ml, respectively. PcrVNH forms a symmetrical peak at 67.3 ml. (C) Linear regression of standard proteins to calculate the molecular weight of PcrVNH. The bar represents the Kav (gel phase-distribution coefficient) value of each protein. The standard curve was calculated as “Kav = 0.980–0.479*LogMw” (R2 = 0.996). The calculated Mw of PcrVNH was ~21.2 KDa. (D) Dynamic light scattering analysis of PcrVNH demonstrated that it forms a symmetrical peak with a diameter of 4.0 nm.