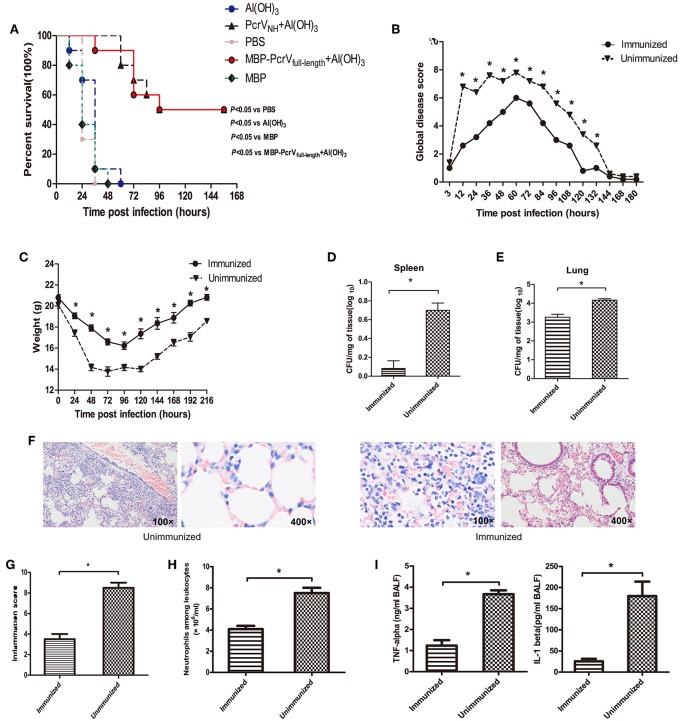
Figure 4.
Vaccination of PcrVNH confers protection in mice. (A) Mice (n = 10) were immunized with Al(OH)3, PcrVNH, PBS, MBP-PcrVfull−length, or MBP. The survival of mice was recorded every 12 h after intratracheal injection with a lethal dose of PA XN-1 for 7 days. (B) The global disease score of mice immunized with PcrVNH after challenge with a sublethal dose of PA XN-1 (n = 5). The score was recorded every 12 h for 8 days. (C) The weight loss of mice immunized with PcrVNH after challenge with a sublethal dose of PA XN-1 (n = 5); the weight was recorded every 24 h for 9 days. (D,E) Assessment of the bacterial load in the lungs and spleen of PcrVNH-immunized mice 24 h after challenge with a sublethal dose of PA XN-1. The bar represents the log number of CFU per mg of spleen (left) and lung (right). (F) HE staining of lungs. PcrVNH-immunized mice were challenged with a sublethal dose of PA XN-1; 24 h later, the lung was collected and stained with hematoxylin and eosin. Images were captured at 100× magnification and 400× magnification. (G) Semiquantitative analysis of lung inflammation. The bar in the right panel represents the inflammation score. (H) Evaluation of neutrophil infiltration in the infected mice. The bars represent the number of neutrophils in 1 ml of BALF of immunized mice 24 h post-challenge. (I) Quantitative measurement of the pro-inflammatory cytokines TNF-α and IL-1β in the lungs. The data are shown as the means ± SE. *P < 0.05, ns, not significant (P > 0.05).