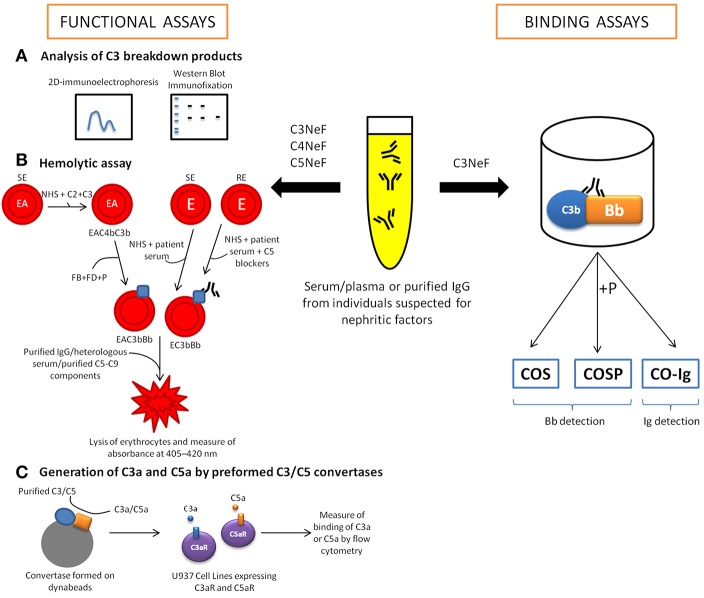
Figure 3.
Diagnostic tools for the detection of nephritic factors (NFs). The functional activity of C3NeF can be determined through quantifying complement activation products (mostly C3 fragments) by two-dimensional immunoelectrophoresis, immunofixation electrophoresis or western blotting (A). However, the main tools for the detection of NFs activity are the hemolytic assays, which measure the lysis of sheep (SE)/rabbit erythrocytes (RE) (B). In these assays, purified complement components are added to perform convertases on the surface SE sensitized with anti-sheep antibodies (EA). Later, purified FB, FD and P are added to form specifically alternative pathway convertase. Other assays are developed using whole serum (patient's serum and normal human serum (NHS), in ratio 1: 1) to generate convertases on the SE or RE surface. Considering that RE could be lysed by human serum, terminal pathway needs to be blocked using C5 blockers (OmCI or eculizumab). Another option to measure NF activity is through the quantification of anaphylatoxins, C3a and C5a (C). There are binding assays to detect C3NeF based on enzyme-linked immunosorbent assays (ELISA) that detect immunoglobulins bound to the preformed convertase complex (CO-Ig). Other versions of the ELISA-based binding assay are the so-called C3NeF stabilization assays, which use a polyclonal antibody to detect plate-bound Bb fragment generated either in the absence (COS assay) or in the presence (COS-P assay) of P.