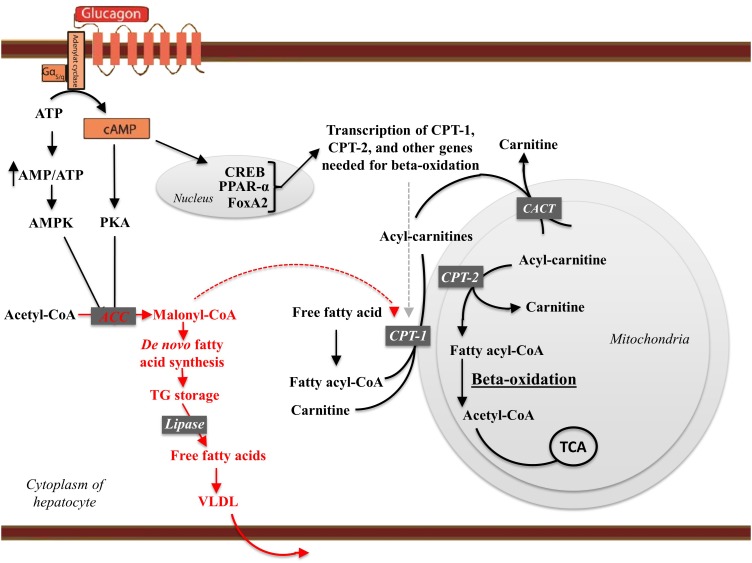
FIGURE 2.
The effects of glucagon receptor signaling on hepatic lipid metabolism. Glucagon activates its cognate receptor, a seven transmembrane receptor coupled to a Gs protein, resulting in AC activity and cAMP production. The increase in intracellular cAMP activates protein kinase A (PKA), which phosphorylates (hence inactivates) acetyl-CoA carboxylase (ACC). Glucagon thus inhibit malonyl-CoA formation and the subsequent de novo fatty acid synthesis. When formed, the fatty acids are, after re-esterification, stored as trigycerides in and released from the hepatocytes in the form of very-low density lipoprotein (VLDL). Thus, glucagon leads the free fatty acids toward beta-oxidation and decreases de novo fatty acid synthesis and VLDL release. cAMP accumulation in hepatocytes activates the cAMP responsible binding element (CREB) protein, which induces the transcription of carnitine acyl transferase-1 (CPT-1), and other genes needed for beta-oxidation. CPT-1 catalyzes the attachment of carnitine to fatty acyl-CoA, forming acyl-carnitine. The acyl-carnitines transverse the mitochondrial membrane mediated via the carnitine-acylcarnitine translocase (CACT). Once in the mitochondrial matrix, carnitine acyl transferase-2 (CPT-2) is responsible for transferring the acyl-group from the acyl-carnitine back to CoA. Carnitine leaves the mitochondria matrix through the carnitine-acylcarnitine translocase. During beta-oxidation, the fatty acid chains are degraded into acetate. Acetate reacts with CoA to yield acetyl-CoA, which reacts with oxaloacetate to form citrate that inhibits glycolysis through inhibition of pyruvate dehydrogenase and phosphofructokinase-1. Finally, citrate enters the citric acid cycle (TCA). Thus, glucagon increases fatty acid catabolism, inhibits glycolysis, and fuels the TCA cycle. By increasing AC activity glucagon increase the AMP/ATP ratio sufficient to activate AMP-activated kinase (AMPK), which phosphorylates ACC, leading to transcriptional activation of peroxisome proliferator-activated receptor-α (PPARα). PPARα stimulates the transcription of genes involved in beta-oxidation including CPT-1, CPT-2, and acetyl-CoA oxidase. Glucagon stimulates FoxA2 activity, which induces transcription of genes such as CPT-1, very-, and medium- long-chain acyl-CoA dehydrogenase. Enzymes and pathways inhibited by glucagon are shown in red, while enzymes and pathways stimulated by glucagon are shown in black.