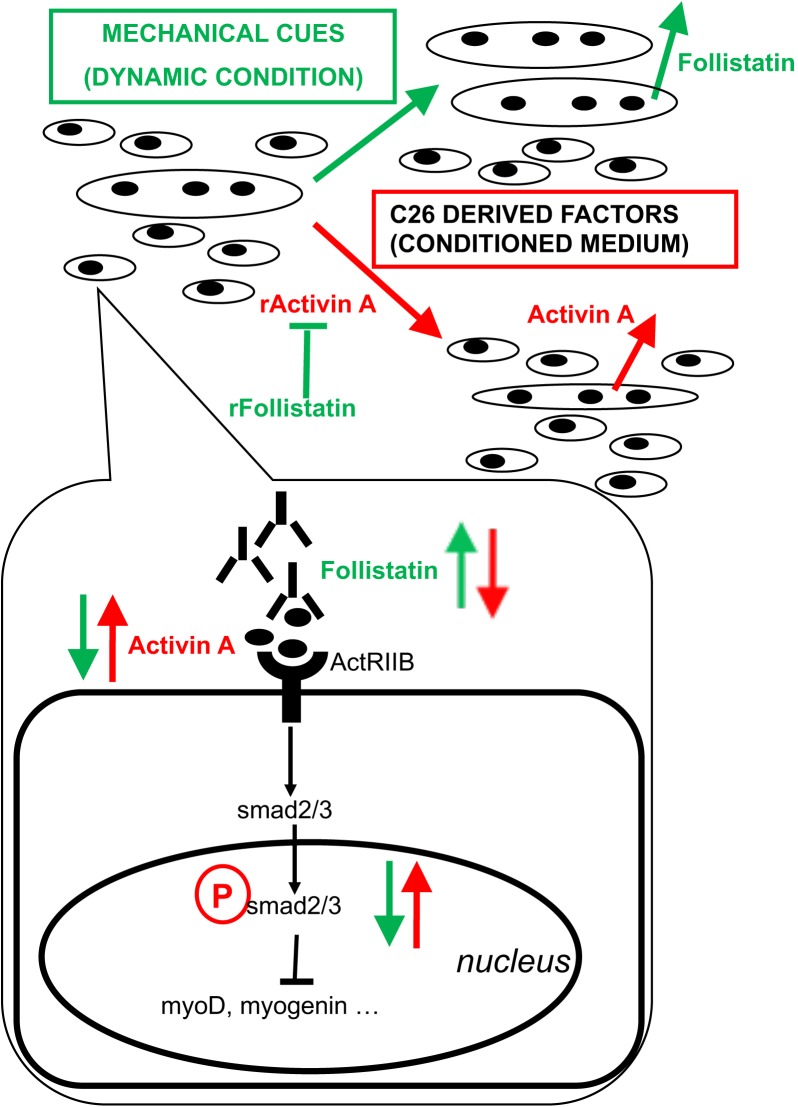
FIGURE 3.
Proposed model of action of tumor-derived factors and mechanical stimulation on myotubes and myoblasts. Mixed cultures of myotubes and myoblasts mature in culture by increasing the diameter of myotubes, the fusion index (i.e., myogenic differentiation tout court, including the formation of novel myotubes) and the number of nuclei per myotube (i.e., myotube accretion by incorporation of myoblasts). C26 tumor-derived factors include activin and induce further expression and release of activin as well as a decrease of follistatin expression and its release by muscle cells, ultimately leading to myotube atrophy, a block of myogenic differentiation and hampered incorporation of myoblasts into myotubes. On the other hand, mechanical stimulation counteracts the negative effects exerted by tumor-derived factors on muscle cells by diminishing the levels of activin available to bind actRIIB: this is obtained by reducing activin concentration in the medium and by rescuing follistatin release by muscle cells. Recombinant activin (rActivin A) mimics tumor CM and its effects are counteracted by recombinant follistatin (rFollistatin). However, rFollistatin only partially counteracts CM: since, in the presence of CM, follistatin rescues the fusion index but not myotube diameter nor the number of NpM, while mechanical stimulation also reverts CM-mediated effects on myotube size, follistatin is mostly responsible for the regulation of myogenic differentiation, while mechanical stimulation preserves myotube size through additional mechanisms. The signaling pathways downstream of actRIIB involve the activation of SMAD2/3 transcriptional activity, which is increased by tumor-derived factors and decreased by mechanical stimulation, resulting in the regulation of MRF expression leading to myoblast differentiation and fusion.