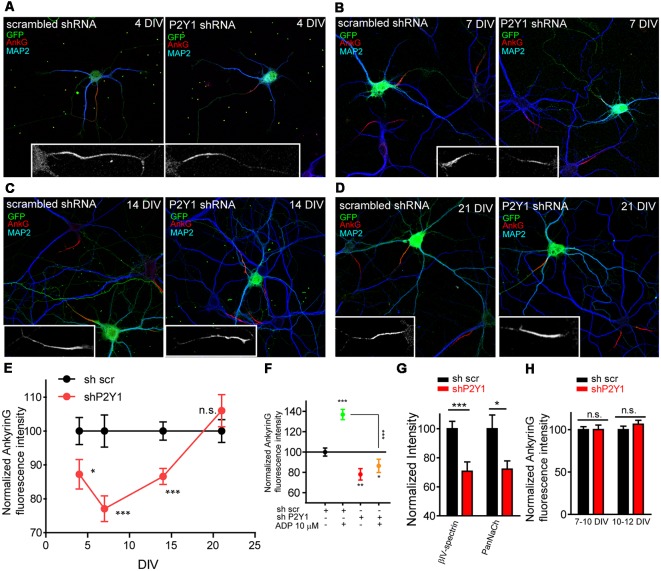
Figure 2.
P2Y1 receptor is necessary to maintain ankyrinG density during early stages of AIS development. (A–D) 4, 7, 14 and 21 DIV hippocampal neurons nucleofected with scrambled and P2Y1 shRNAs. After nucleofection neurons were kept in culture for 4 (A), 7 (B), 14 (C) and 21 days (D) and stained with MAP2 (blue) and ankyrinG (red) antibodies. Nucleofected neurons were identified by GFP expression (green). Inserts show magnifications of AISs (gray) of each neuron. (E) Normalized ankyrinG intensity at different days post-nucleofection with scrambled shRNA (shscr, black) or P2Y1 shRNA (shP2Y1, red). shP2Y1 data are normalized to their respective shscr controls at each developmental stage. *p < 0.05, **p < 0.01, ***p < 0.001, two-tail t-test. (F) Normalized ankyrinG intensity in 10 DIV neurons expressing shscr or shP2Y1 plasmids, treated with vehicle or ADP from 7 to 10 DIV. *p < 0.05, **p < 0.01, ***p < 0.001, two-tail t-test. Data were acquired from three independent experiments (30 neurons/experimental condition in each experiment). (G) βIV-spectrin and voltage-gated sodium channels (PanNaCh) normalized intensity in 7 DIV control shscr and shP2Y1 expressing neurons. *p < 0.05, ***p < 0.001, two-tail t-test. Data were acquired from three independent experiments (at least 25 neurons/experimental condition in each experiment). (H) Normalized ankyrinG intensity in 10 and 12 DIV hippocampal transfected at 7 or 10 DIV, respectively, to express shscr or shP2Y1 interference RNAs. Data were acquired from three independent experiments (at least 20 neurons/experimental condition in each experiment). n.s., not significant, two tail t-test. Data in graphs are represented as the mean ± SEM.