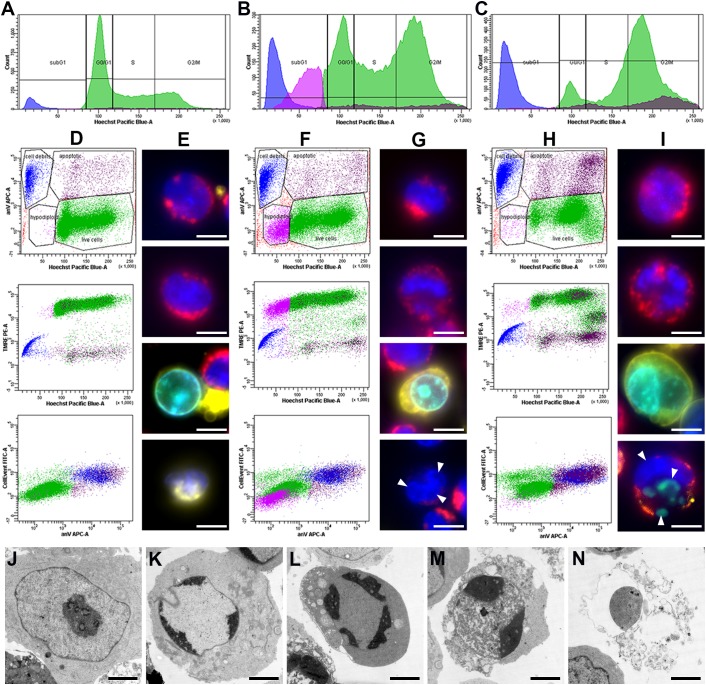
FIGURE 2.
RPMI8866 B-lymphocytes untreated (control) and treated with paclitaxel (10 and 1000 nM) for 24 h. (A–C) DNA content curves in individual samples assessed by flow cytometry after Hoechst33342 staining and linear gates set to determine sub-G1, G0/G1, S, and G2/M cell cycle populations [colored subpopulations correspond to live cells (green), apoptotic cells (brown), hypodiploid cells (purple) and cell debris (blue) as gated on dot plots (D–F)]. 50 000 events were analyzed per sample. (A) Normal cell cycle distribution in control cells; G2/M comprises 18% cells, sub-G1 comprises 6% cells. (B) G2/M accumulation after 10 nM paclitaxel treatment; G2/M comprises 30% cells, sub-G1 comprises 26% cells. (C) G2/M accumulation after 1000 nM paclitaxel treatment; G2/M comprises 49% cells, sub-G1 comprises 19% cells. (D,F,H) Dot plots representing simultaneous staining of RPMI8866 B-lymphocytes with Hoechst33342, TMRE, annexin V-Alexa Fluor 647, and CellEvent. Region gates are set to determine live cells (green), apoptotic cells (brown), hypodiploid cells (purple) and cell debris (blue) based on Hoechst33342 vs. annexin V staining. (D) Normal apoptosis rate in control cells. Region gates are set to live cells (green) – 89.4%, apoptotic cells (brown) – 3.2%, hypodiploid cells (purple) – 0.6% and cell debris (blue) – 6.8% based on Hoechst33342 vs. annexin V staining. The colors of the gated populations remain the same in the TMRE vs. Hoechst33342 and CellEvent vs. annexin V graphs. (F) G2/M and sub-G1 accumulation after 10 nM paclitaxel treatment. Region gates are set to live cells (green) – 69.0%, apoptotic cells (brown) – 4.9%, hypodiploid cells (purple) – 13.5% and cell debris (blue) – 12.6% based on Hoechst33342 vs. annexin V staining. The colors of the gated populations remain the same in the TMRE vs. Hoechst33342 and CellEvent vs. annexin V graphs. (H) G2/M accumulation after 1000 nM paclitaxel treatment. Region gates are set to live cells (green) – 67.0%, apoptotic cells (brown) – 13.3%, hypodiploid cells (purple) – 1.6% and cell debris (blue) – 18.1% based on Hoechst33342 vs. annexin V staining. The colors of the gated populations remain the same in the TMRE vs. Hoechst33342 and CellEvent vs. annexin V graphs. (E,G,I) Fluorescence images of cells untreated (control) and treated with paclitaxel (10 and 1000 nM) for 24 h. Simultaneous staining of RPMI8866 B-lymphocytes with Hoechst33342 (blue), TMRE (red), annexin V-Alexa 647 (yellow), and CellEvent (green). Scale bar – 10 μm. (E) Control cells; top two images correspond to live cells with bright nuclear Hoechst33342 and mitochondrial TMRE fluorescence, bottom two images correspond to an apoptotic cell with CellEvent staining in the nucleus and surface annexin V and cell debris with a dim nucleus and surface annexin V. (G) 10 nM paclitaxel treatment; top two images correspond to live cells with bright nuclear Hoechst33342 and mitochondrial TMRE fluorescence, third image corresponds to an apoptotic cell with CellEvent staining in the nucleus and surface annexin V, bottom image corresponds to aneuploid (hypodiploid) cell with dim mitochondrial TMRE fluorescence and micronuclei. White arrowheads indicate micronuclei. (I) 1000 nM paclitaxel treatment; top two images correspond to live cells with bright nuclear Hoechst33342 and mitochondrial TMRE fluorescence, bottom two images correspond to apoptotic cells with CellEvent staining in the nucleus and surface annexin V with/without micronuclei. White arrowheads indicate micronuclei. (J–N) Electron microscopy of cells at various stages of apoptosis after 10 nM paclitaxel treatment. Scale bar – 2 μm. (J) Normal cell with typical RPMI8866 lymphocyte morphology. (K) Chromatin marginalization. (L) Decrease of cell volume and condensation of cytoplasm (cell shrinkage). (M) Early stage of cell destruction (secondary necrosis). (N) Cellular debris inside which the residual chromatin and cytoplasmic organelles can be distinguished.