Table 4.
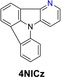
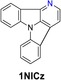
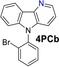
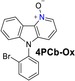
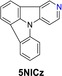
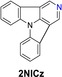
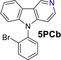
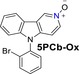
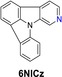
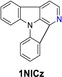
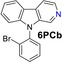
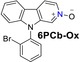
C−H activation towards mono‐substituted NICzs starting from carboline precursors.
| Precursor |
t
[h] |
Product and yield [%][a] | |
|---|---|---|---|
|
|
||
|
6 | n.d.[b]/(15) | n.d.[b]/(45) |
|
8 | 7/(3) | 61/(58) |
|
|
||
|
4 | 11/(0) | 76/(46) |
|
4 | 14/(4) | 62/(44) |
|
|
||
|
6 | 74/(61) | 19/(16) |
|
4 | 16/(12) | 71/(57) |
[a] Overall yield of ring‐closed products (ratio determined by 1H NMR) and yields of isolated material in brackets. For reactions starting from N‐oxides, yields over two steps (C−H activation and reduction) are given. [b] Not determined owing to overlapping signals from small amounts of dehalogenated byproduct.
