Figure 2.
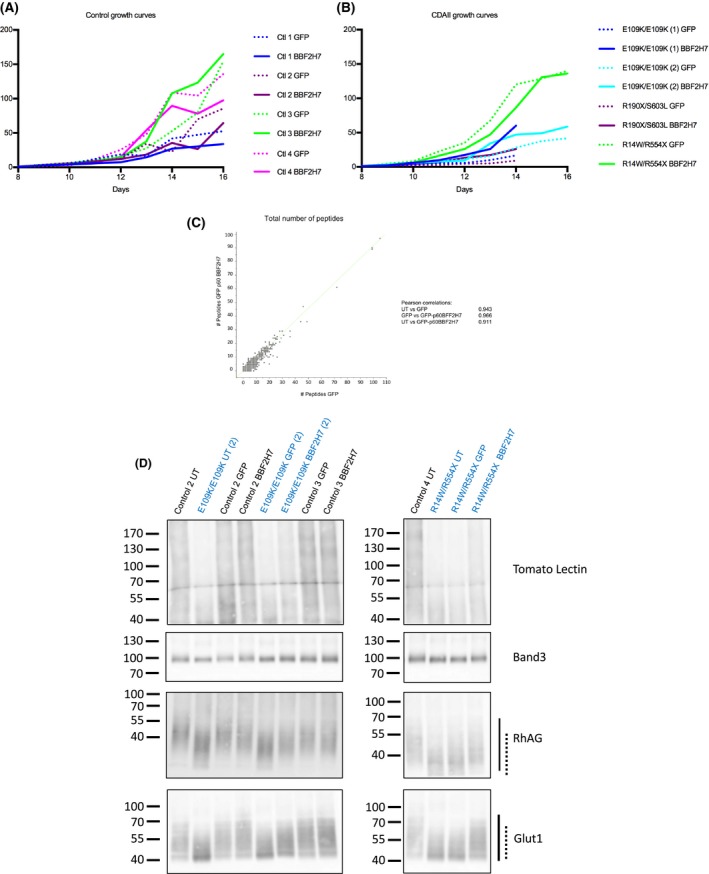
Effects of GFP‐p60‐BBF2H7 expression on in vitro erythropoiesis. (A) Growth curves of healthy control cells (n = 4). Dotted lines are used for GFP‐expressing cells and same colour solid lines are used for cells from the same donor expressing GFP‐p60 BBF2H7. (B) Growth curves of CDAII patient cells (4 cultures from 3 CDAII patients). Dotted lines are used for GFP expressing cells and same colour solid lines are used for cells from the same donor expressing GFP‐p60 BBF2H7. (C) Scatter plot of label free peptide numbers for each of the 2002 proteins identified by mass spectrometry using 2 × 106 in vitro reticulocytes obtained from GFP versus GFP‐p60 BBF2H7 expressing erythroblasts, derived from the same healthy control donor. 2 × 106 reticulocytes filtered from each of 3 cultures, derived from the same control healthy donor (un‐transduced, GFP‐expressing or GFP‐p60 BBF2H7‐expressing cells) were used for proteomics analysis of whole cell lysates and the data was analysed using the Perseus software (Max Planck Institute, Planegg, Germany). In total, 2002 proteins were identified, and the Pearson correlation showed that there was no significant difference between any of the 3 cultures (Pearson correlations for UT versus GFP: 0.943; GFP versus BBF2H7: 0.966; UT versus BBF2H7: 0.911). (D) Western blotting of in vitro reticulocyte lysates using Tomato Lectin or antibodies against highly glycosylated membrane proteins: Band3, RhAG and Glut1. The overall hypo‐glycosylation of reticulocyte lysates derived from CDAII and control cells (un‐transduced, GFP‐ or GFP‐p60‐BBF2H7 expressing cells) is visualised by using Tomato Lectin (top panel; controls (black writing) and 2 cultures of CDAII patients (blue writing)). Hypo‐glycosylation of specific membrane proteins (i.e. Band3, RhAG or Glut1) is shown below by the protein's mobility shift. Hypo‐glycosylation of RhAG and Glut1 is evident when comparing control to CDAII reticulocyte lysates; RhAG and Glut1 glycosylation is partially restored in CDAII by GFP‐p60 BBF2H7 expression. The solid lines on the side of the blots show the mobility of the glycosylated protein in healthy controls whereas the dotted lines show the mobility of the hypo‐glycosylated form as seen in CDAII cells.
