Abstract
Dexamethasone-phosphate is widely used for intratympanic therapy in humans. We assessed the pharmacokinetics of dexamethasone entry into perilymph when administered as dexamethasone-phosphate solution or as a micronized dexamethasone suspension, with and without inclusion of poloxamer gel in the medium. After a 1-hour application to guinea pigs, 10 independent samples of perilymph were collected from the lateral semi-circular canal of each animal, allowing entry at the round window and stapes to be independently assessed. Both forms of dexamethasone entered perilymph predominantly at the round window (73%) with a lower proportion entering at the stapes (22%). When normalized by applied concentration, dexamethasone-phosphate was found to enter perilymph far more slowly than dexamethasone, in accordance with its calculated lipid solubility and polar surface area properties. Dexamethasone-phosphate therefore has a problematic combination of kinetic properties when used for local therapy of the ear. It is relatively impermeable and enters perilymph only slowly from the middle ear. It is then metabolized in the ear to dexamethasone, which is more permeable through tissue boundaries and is rapidly lost from perilymph. Understanding the influence of molecular properties on the distribution of drugs in perilymph provides a new level of understanding which may help optimize drug therapies of the ear.
Keywords: dexamethasone, dexamethasone-phosphate, lipid solubility, polar surface area, round window, stapes, permeability, perilymph, elimination, intratympanic therapy
1. Introduction
When physicians treat the inner ear with local, intratympanic applications of “dexamethasone” solution it is generally administered in the form of dexamethasone-phosphate. The phosphate group is covalently bonded to dexamethasone and does not dissociate in aqueous solution. Rather, dexamethasone-phosphate is a divalent anion, available as a disodium salt. Dexamethasone-phosphate (FW 470.4) is a pro-drug and is inactive until the phosphate group (approximately 20% of the molecule) is cleaved by phosphatases in the body to produce dexamethasone (FW 392.5). The rationale for the use of dexamethasone-phosphate over dexamethasone for systemic therapy was largely based on its aqueous solubility. The phosphate group makes the molecule more polar, increasing aqueous solubility by more than 500x, from 89 μg/mL for dexamethasone to 50 mg/mL for dexamethasone-phosphate. This allows a higher dose to be given with a lower injected volume. When applied systemically, the phosphate is cleaved by phosphatases in the liver, by which time the drug is diluted to a degree that the dexamethasone remains in solution. Intratympanically-administered dexamethasone-phosphate is used clinically to treat Meniere’s disease and idiopathic sudden sensorineural hearing loss [Schoo et al, 2017; Plontke et al., 2009]. It is usually injected at concentrations of 4 – 12 mg/mL [review and meta-analysis by Liebau et al., 2017]. Some studies have now suggested that a higher concentration, 24 mg/mL may be more effective in the treatment of idiopathic sudden sensorineural hearing loss [Alexander et al., 2015; Hobson et al, 2016].
Dexamethasone has also been administered to the ear in its native, less soluble form. It is delivered as a suspension of micronized particles. A formulation made up of a dexamethasone suspension in poloxamer 407 gel has been developed and is currently in Phase 3 clinical trials for the treatment of patients with Meniere’s disease [Lambert et al. 2016]. In animals, a similar formulation was shown to establish therapeutic concentrations of dexamethasone in perilymph for up to 28 days [Wang et al., 2009; Piu et al 2011].
A number of key molecular properties play a pivotal role in how substances pass through biological barriers, specifically their lipid solubility and the polar properties of the molecule [Egan et al., 2000]. One important parameter is the lipid solubility of the molecule, originally determined as the octanol/water partition coefficient. There are now computational methods to calculate the lipid partition coefficient from molecular structure. The PubChem website (https://pubchem.ncbi.nlm.nih.gov) provides XlogP3 values [Cheng et al., 2007] while other sites such as the SwissADME website (http://www.swissadme.ch) calculate a similar parameter, WLOGP [Daina et al., 2017]. A second important property is characterized by measures of the polar properties of the molecule. Topological Polar Surface Area is calculated, abbreviated as TPSA or TPSA/A2, which takes into account the extent and presence of polar, charged groups on the molecule. These two parameters (WLOGP and TPSA) combined have been shown to provide an indication of how readily drugs pass through common biological barriers, such as the blood brain barrier and the gut [Daina & Zoete, 2016]. This is because many biological barriers are formed by sheets of epithelial or endothelial cells with tight intercellular junctions. Passive passage of small molecules through such boundaries depends on their ability to pass though the lipid bilayer of the cell membranes forming the boundary. These and other parameters relevant to pharmacokinetics can now be calculated for any molecule using the SwissADME website.
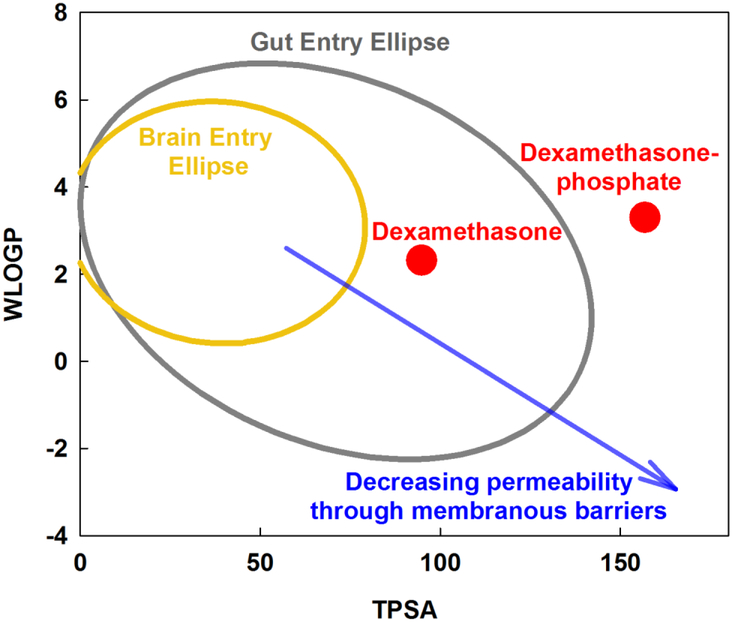
The calculated parameters for the two forms of dexamethasone used in this study are shown in Figure 1. The graph shows WLOGP (y-axis) plotted against TPSA (x-axis) and has been described as an “egg plot” [Daina & Zoete, 2016]. The yellow ellipse (egg yolk) defines the boundary of properties that allow molecules to pass through the blood-brain barrier, based on a dataset of 260 molecules for which entry into the human brain was measured. The gray ellipse (egg white) delimits the range of properties that allow molecules to permeate the human gut, based on a dataset of 600 molecules. In general, small, nonpolar (low TPSA) lipophilic (high WLOGP) molecules pass through membranous boundaries while large, polar (high TPSA) hydrophilic (low WLOGP) molecules pass less easily. On this plot, it is apparent that the addition of the polar phosphate group to dexamethasone substantially changes its calculated molecular properties. Dexamethasone-phosphate would be expected to be less permeable through biological barriers than dexamethasone. The analysis shows that the exact form of the drug administered potentially has an important influence on kinetics and therefore potentially on efficacy.
Figure 1:
Molecular properties compared for two forms of dexamethasone. WLOGP is a measure of lipid solubility of the drug and TPSA is a measure of the surface area of the molecule occupied by charged groups (see text). Molecules with higher TPSA (large, polar) and lower WLOGP (hydrophilic), i.e. at the bottom right of the plot, typically have high aqueous solubility but do not readily pass through biological membranous boundaries. Molecules with low TPSA (small, nonpolar) and high WLOGP (lipophilic) i.e. at the top left of the plot are typically less soluble but pass more readily through membranous boundaries. The yellow ellipse (egg yolk) indicates the range of properties for molecules that pass through the blood brain barrier, while the gray ellipse (egg white) indicates the range of properties for molecules that pass into the body through the gut (Daina & Zoete, 2106). Dexamethasone-phosphate is larger and more polar than dexamethasone, giving it higher aqueous solubility but predicting it will pass less readily through membranous boundaries.
In the present study, we have measured and compared the entry of dexamethasone-phosphate and dexamethasone in guinea pigs, using computer simulations of the data to interpret the findings quantitatively.
2. Materials and Methods
Animals
The study used 16 pigmented, NIH-strain guinea pigs weighing 400 – 600 g following policies in accordance with the United States Department of Agriculture and National Institutes of Health guidelines for the handling and use of laboratory animals. Experiments were performed under protocol 20160053, approved by the Institutional Animal Care and Use Committee of Washington University.
Animals were first anesthetized with 100 mg/kg sodium thiobutabarbital (Inactin, Sigma, St Louis, MO) and then maintained on 0.8 to 1.2 % isofluorane in oxygen using a mechanical ventilator coupled to a tracheal cannula. End-tidal CO2 level was monitored (CapnoTrue AMP Bluepoint Medical, The Netherlands) and maintained close to 5% by adjustment of the ventilator tidal volume. Heart rate and oxygen saturation were monitored with a pulse-oximeter (Surgivet. Waukesha, WI). A thermistor-controlled heating blanket (Harvard Apparatus, Holliston, MA) was used to maintain body temperature at 38 °C.
Drug delivery and perilymph sampling were accomplished with a lateral exposure of the auditory bulla, through an incision posterior to the ear canal. The bulla was opened allowing the round window (RW) niche and the bone overlying the lateral semi-circular canal (LSCC) to be visualized.
Preparation for Perilymph Sampling
Before any drug was applied, the LSCC was first prepared for perilymph sampling by thinning the overlying bone with a dental burr. A layer of cyanoacrylate glue (Permabond 101; Permabond, Pottstown, PA) was applied to the thinned bone, followed by a covering of two-part silicone adhesive (Kwik-Cast, World Precision Instruments, Sarasota, FL), built up at the edges to form a hydrophobic cup. This allowed perilymph to be collected free of contamination of drug solution subsequently applied to the RW niche.
Dexamethasone Delivery
Drug solution was applied to the RW niche with a hand-held micropipetter. A 20 μL volume was applied, sufficient to fill the entire niche and stapes area of the guinea pig. A single application of drug solution was applied for one of four conditions.
4 mg/mL dexamethasone sodium phosphate (Fresenius Kabi, Lake Zurich, IL, USA), n=4.
4 mg/mL dexamethasone sodium phosphate with added 17% Poloxamer 407 (P1166, Spectrum Chemicals, Gardena CA, USA), n=4.
4.5% micronized dexamethasone (DE121, Spectrum Chemicals) in phosphate-buffered saline (PBS), n=4. The measured dissolved concentration in the supernatant after centrifugation of this suspension was 94.2 μg/mL.
4.5 % micronized dexamethasone + 17% Poloxamer 407 in PBS, n=4. Poloxamer increases the solubility of hydrophobic compounds and when present in the medium. The dissolved concentration in the supernatant after centrifugation of this suspension was 148 μg/mL.
Perilymph was sampled from the LSCC 60 mins after the application to the RW niche using the procedure described below. This sampling time allowed entry at the RW and stapes to be differentiated before being clouded by the effects of inter-scala communication between scala tympani and scala vestibuli. It also minimized the influence of the rapidly declining middle ear concentration when drug was applied as a solution, specifically in the case of dexamethasone-phosphate. For experiments in which dexamethasone-phosphate was applied, a sample of the fluid remaining in the RW niche was collected for analysis after perilymph had been sampled. The average time for collecting this sample was 93 min after the solution had been applied.
Sequential Sampling from the Lateral Semi-Circular Canal
The technique of sequential sampling, and its value to quantify drug gradients in the perilymphatic spaces, has been described elsewhere [Mynatt et al., 2006, Salt et al., 2012]. Sampling commenced by making a 30–40 μm fenestration in the thinned and silicone-coated bony wall of the LSCC with a 30° House stapes pick (N1705 80, Bausch and Lomb Inc.). As the clear fluid accumulated on the hydrophobic surface of the silicone it was collected with hand-held, blunt tipped capillary tubes (VWR 53432–706). The exact volume of each sample was established by measuring its length with a calibrated dissecting microscope. Perilymph was collected from the LSCC as 20 individual 1 μL samples. As perilymph leaks from the perforated LSCC at about 0.5 – 1 μL/min, it takes between 20 and 40 minutes to collect the 20 samples. The 20 individual samples collected in this manner were paired and each pair added to 50 μL of 1:1 methanol:water diluent, resulting in 10 diluted samples (2 μL perilymph + 50 μL dilutent). Samples were stored frozen at −80°C until analysis. Samples were analyzed using high-pressure liquid chromatography combined with mass spectrometry detection [Wang et al., 2011]. Analysis operators were blinded to the experimental conditions and to the origin of the samples. The LLOQ for dexamethasone was about 0.2 ng/mL and the LLOQ for dexamethasone-phosphate was 20 ng/mL after an additional dilution factor of 2. In experiments where dexamethasone was applied only dexamethasone was measured. In experiments where dexamethasone-phosphate was applied, both dexamethasone and dexamethasone-phosphate were measured.
Quantitative Interpretation of Sequential Sample Data through Simulations
A computer program simulating the inner ear fluids (available for download at http://oto.wustl.edu/saltlab) replicated most aspects of the experiments performed here in great detail. For intratympanic injections, calculations include the known entry sites into perilymph of the guinea pig, which includes the round window, stapes and apex. Drug distribution is calculated throughout the fluid and tissue spaces of the cochlea and vestibular system, based on diffusion coefficients and elimination rates that can be varied. The calculations use the cross-sectional area of each fluid and tissue compartment, each quantified at 0.1 mm intervals along their length. Simulation of the sequential sampling procedure includes the fluid flows during sampling according to the specific volumes and collection times for each of the samples. Simulations were fitted to the measured perilymph data by adjusting entry permeability at the RW, stapes and apex until calculated sample concentrations matched the measured data. Stapes permeability was adjusted to match samples 2–3, entry through the apical bone (a minor component) was adjusted to match samples 4–6, and RW membrane permeability was adjusted to match samples 7–9.
Statistical significance of measurements was assessed using Sigmaplot v13 software (Systat: systatsoftware.com).
3. Results
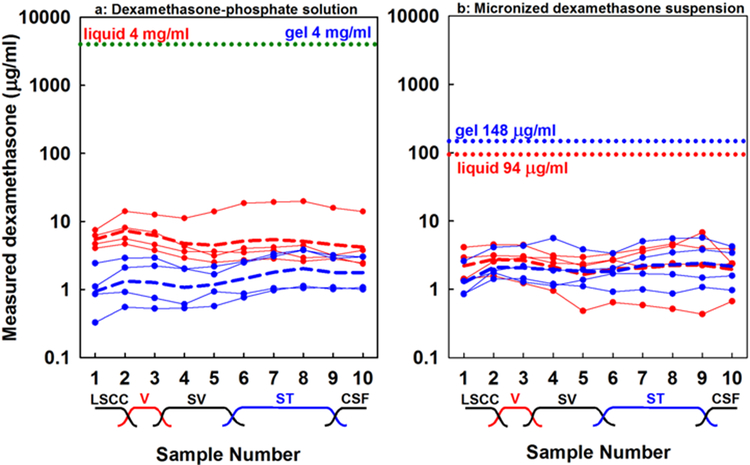
The measured perilymph concentrations resulting from RW niche applications of dexamethasone-phosphate solution (4 mg/mL) or dexamethasone suspension (4.5%) are shown in Figure 2. Both forms were administered in either liquid form (red curves) or in Poloxamer 407, which gels at body temperature (blue curves). The applied concentration for dexamethasone-phosphate was 4 mg/mL in both liquid and gel forms and for dexamethasone was 94.2 μg/mL when in liquid form and 148 μg/mL in the gel, as indicated on the figure. The initial samples collected originate from the semi-circular canal and vestibule, with concentrations that reflect entry through the stapes. Later samples originate from scala tympani and reflect entry at the RW membrane. It is strikingly apparent in the figure that the perilymph concentrations achieved with the two forms of dexamethasone are remarkably similar, even though dexamethasone-phosphate was applied at ~35x higher concentration than dexamethasone. Two-way ANOVA of the logarithms of perilymph dexamethasone-phosphate concentrations showed that perilymph reached significantly higher concentration when applied in liquid form (Mean 5.19 μg/mL) compared to when applied in poloxamer gel (Mean 1.41 μg/mL) (ANOVA, Bonferroni t-test; t-8.09, p<0.001). In contrast, dexamethasone in perilymph did not differ significantly when applied in liquid form (Mean = 2.16 μg/mL) or when applied in poloxamer gel (Mean = 1.97 μg/mL) (ANOVA Bonferroni t-test, t=0.56, p=1).
Figure 2:
Concentrations of sequential perilymph samples taken from the lateral semi-circular canal 60 mins after Intratympanic application of one of the two forms of dexamethasone. (a) Dexamethasone-phosphate solution. (b) Micronized dexamethasone suspension. In both cases, red lines indicate measurements when drug was applied as a liquid solution and blue lines indicate measurements when drug was applied in Poloxamer gel. Each curve with symbols shows the measured samples from a single animal. Applied drug concentrations are indicated by dotted lines. Dashed lines show the group means, calculated from the logarithms of sample concentrations. The approximate anatomic origins of the samples are indicated. LSCC: lateral semi-circular canal; V: vestibule; SV: scala vestibuli. ST: scala tympani; CSF: cerebrospinal fluid. Samples 2–3 originate from the vestibule and reflect drug entry through the stapes. Samples 7–9 originate from scala tympani and reflect entry at the round window membrane. Even though the applied concentration of dexamethasone-phosphate was far higher, perilymph levels achieved were relatively similar for both applied forms.
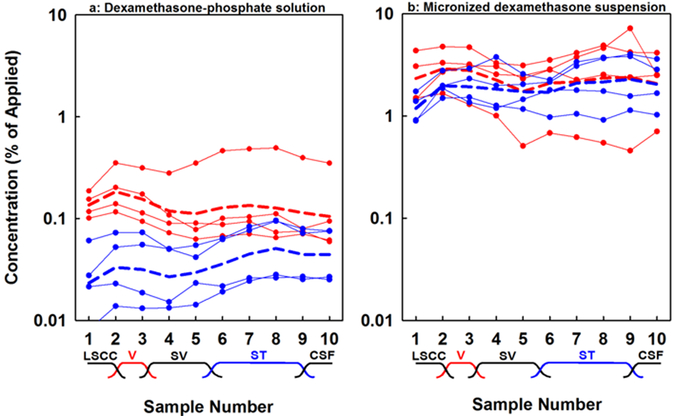
The same data, normalized by the applied concentration measured for each experiment are shown in Figure 3. Perilymph dexamethasone, as indicated by averaging all 40 samples for a given condition, reached 0.13 % of the applied concentration when dexamethasone-phosphate was given in liquid form and 0.036 % when given in gel. In contrast, perilymph dexamethasone levels reached 2.31 % of the applied concentration when given as a suspension in liquid and 1.90% when given in gel. Normalized perilymph levels were 18x higher for dexamethasone compared to dexamethasone-phosphate when given in liquid form and 52x higher when in gel form. In semi-quantitative terms, this shows that dexamethasone enters perilymph far more readily than dexamethasone-phosphate, consistent with the differences in their molecular properties shown in Figure 1.
Figure 3:
Normalized perilymph concentrations derived from the data presented in Figure 2. (a) Dexamethasone-phosphate solution applied. (b) Dexamethasone suspension applied. In both cases, red curves show results when drug was applied as a liquid. Blue curves show results when drug was applied in Poloxamer 407 gel. Curves with symbols show individual experiments. Dashed lines show the group means, calculated using the logarithms of sample concentrations. When in liquid form, dexamethasone-phosphate enters perilymph far more slowly than dexamethasone and the difference even greater when applied in Poloxamer gel. The lower rate of entry for dexamethasone-phosphate is in agreement with the calculated physical properties of the drugs shown in Figure 1.
Within the ear, dexamethasone-phosphate is metabolized to its active form, dexamethasone. In the analysis of perilymph samples from ears administered dexamethasone-phosphate we measured both forms of dexamethasone. The data presented in Figures 2 and 3 represent the combined total of both forms. In 7 of the 8 experiments administering dexamethasone-phosphate, the phosphate form was unmeasurably low in perilymph and only dexamethasone was present. In only one case (the highest curve on the figure) was dexamethasone-phosphate detectable, amounting to 18 % of the total measured. As the vast majority of drug measured in perilymph was found to be in the form of dexamethasone, our analysis with computer simulations therefore used diffusion and pharmacokinetic parameters for dexamethasone, rather than dexamethasone-phosphate. Kinetic parameters for dexamethasone were derived by re-analyzing prior sampling data [Salt et al., 2012] using more recent estimates of perilymph-CSF exchange and perilymph flow in the intact cochlea, established with fluorescent dextran marker experiments [Salt et al., 2015]. From this re-analysis, elimination half-times were determined to be 46 min for scala tympani and 91 min for scala vestibuli, which is slower than the previous estimates which did not include CSF dilution effects [Salt et al., 2012].
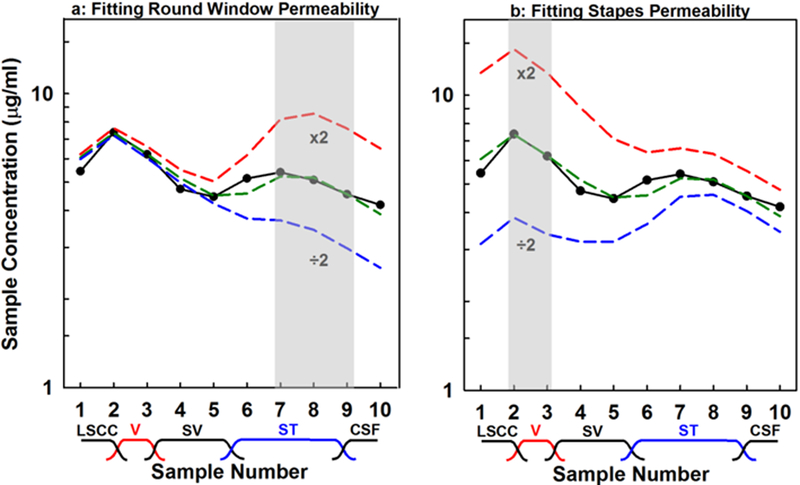
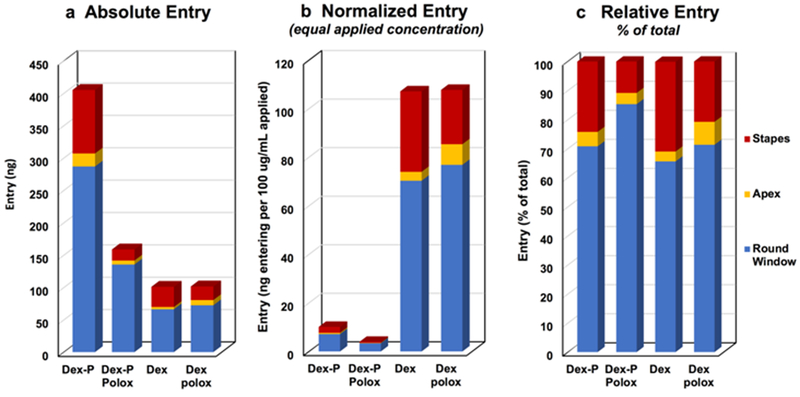
Computer simulations of the present data were used to provide a quantitative interpretation of the entry from the middle ear to perilymph. The mean curve for each of the four groups was simulated, using sample volumes and times averaged for the experiments of the group. Permeability values corresponding to the RW membrane, stapes and apical bone were adjusted to best fit the measured sample data. For dexamethasone-phosphate applied as a liquid the fitting procedure is illustrated in Figure 4. The figure shows the best fit curve (green dashed line) and also for two other conditions; when permeability is doubled or halved for the RW membrane (Figure 4a) or for the stapes (Figure 4b). For the RW, we compared samples 7–9 from the model with the measured data, as indicated by the shaded box. Stapes entry was adjusted by comparing samples 2–3 of the model with the measured data. Some entry is also known to occur through the thin bone at the apex of the guinea pig [Mikulec et al., 2009; Salt et al., 2011]. Apical entry was adjusted by comparing samples 4–6, but entry at this site was generally small (< 5% of total entry), so it is not shown here. The entry parameters and amounts of entry for the 4 experimental conditions derived from this analysis are given in Table 1 and compared graphically in Figure 5. Entry amounts are those derived by simulation to occur during the 60 min application prior to the perilymph sampling procedure which itself influences entry as perilymph concentrations are altered by sampling. In absolute terms, entry was highest for dexamethasone-phosphate liquid solution, but when normalized by the applied concentration, entry was highest for dexamethasone suspensions.
Figure 4:
Summary of how calculated sample curves are fitted to measured data (black curve), in this case for dexamethasone-phosphate in liquid form. (a) The variable representing round window permeability in the model is adjusted to match calculated samples 7–9 to the measured values, which in this case required a value of 28.0 × 10−9 m/s. The influence of doubling and halving the permeability are also shown. (b) Similar adjustment of the variable representing stapes permeability to match calculated samples 2–3 to the measured data. Subsequent samples are influenced by the change, because they have to pass through the vestibule during the sequential sampling procedure.
Table 1:
Dexamethasone entry parameters derived from simulation of data
| Dexamethasone-phosphate liquid |
Dexamethasone-phosphate gel |
Dexamethasone suspension liquid |
Dexamethasone suspension gel |
|
|---|---|---|---|---|
| Round Window permeability X 10−9 m/s |
28.0 | 13.1 | 150 | 165 |
| Stapes permeability X 10−9 m/s |
560 | 97.5 | 4000 | 2680 |
| Round window entry (ng) | 286 | 135 | 66.2 | 72.4 |
| Stapes entry (ng) | 98.2 | 172 | 31.3 | 21.1 |
Figure 5:
Dexamethasone entry derived by simulations of the data viewed from three perspectives. (a) Absolute entry shows the total amount (in ng) entering at the round window (blue), apex (yellow) and stapes (red) for the 4 experimental conditions of the study. The amount was substantially higher for dexamethasone-phosphate (Dex-P) liquid solution than for all other conditions. (b) When normalized by the applied concentration entry was far higher for dexamethasone (Dex) than dexamethasone-phosphate. (c) Normalizing each condition to the total amount entering allowed the three sites of entry to be best compared. Entry at the stapes appeared to be notably lower when dexamethasone-phosphate was applied in gel.
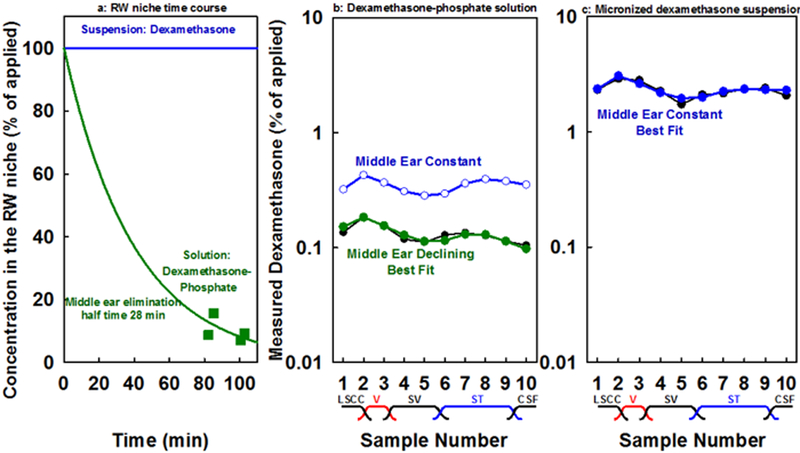
Another important factor influencing the analysis concerns differences in middle ear kinetics for dexamethasone-phosphate solutions compared to dexamethasone suspensions. Measurements of the fluid samples taken from the RW niche after perilymph sampling (at an average of 93 min after application) in experiments where dexamethasone-phosphate was applied, showed the concentration had fallen substantially to 0.41 mg/mL (SD 0.15; n=4; ~10% of the applied concentration) for applications in liquid and to 0.73 mg/mL (SD 0.21; n=4; ~18% of the applied concentration) for applications in gel. Furthermore, in both conditions a substantial amount of the applied dexamethasone-phosphate had been metabolized, with dexamethasone amounting to 71% of the total amount in the niche when dexamethasone-phosphate was applied in liquid but less, 54% of the total in the niche, when applied as gel. It was not possible to measure the dissolved concentrations for dexamethasone suspensions in the RW niche as the available volume was too small to be able to separate supernatant from undissolved drug. Nevertheless, as there was a substantial amount of undissolved drug in the RW niche at the end of experiments, it can be assumed that the dissolved concentration in the niche was maintained through the relatively brief experimental period used here. The decline of dexamethasone-phosphate in the RW niche with time raises a concern that part of the reason why normalized perilymph levels were low with dexamethasone-phosphate applications was because they were normalized by the initially-applied concentration, rather than considering the declining middle ear concentration with time, which is the driving force for entry into perilymph. The simulations above did take the declining middle ear concentration into account, by including a component representing elimination from the middle ear with a half time of 28 min, as shown in Figure 6a. They also allowed the influence of middle ear kinetics on perilymph levels to be quantified. Figure 6b and Figure 6c show fits to the normalized measured data with dexamethasone-phosphate or dexamethasone applications. The solid symbols show calculated samples using the parameters established in Figure 4, in which middle ear concentration was declining with time for dexamethasone-phosphate and was maintained constant with time for dexamethasone. The curve with open symbols shows the calculated curve with the same entry parameters but in this case holding the middle ear concentration constant. It is apparent that the declining middle ear concentration does have a sizeable influence on perilymph concentration but permeability differences still play the dominant role causing the differences between dexamethasone-phosphate and dexamethasone.
Figure 6:
(a) Time course of concentration change in the round window niche used for calculations, based on the concentration measured for fluid from the round window niche taken after perilymph sampling (green symbols). (b) The best fit curve (solid green symbols) to perilymph measurements (black line) when dexamethasone-phosphate was applied. The calculations incorporated the declining middle ear concentration from (a). Blue open circles show calculated sample concentrations with the same entry parameters but with middle ear concentration held constant, rather than declining (c) The best fit for dexamethasone (solid blue symbols) calculated for a constant concentration in the middle ear. The analysis confirms that declining middle ear concentration makes a contribution to the lower perilymph levels measured with dexamethasone-phosphate applications but does not fully account for the differences between the two drugs. Even allowing for differences in middle ear kinetics, normalized dexamethasone levels were still over 6x lower when dexamethasone-phosphate was applied
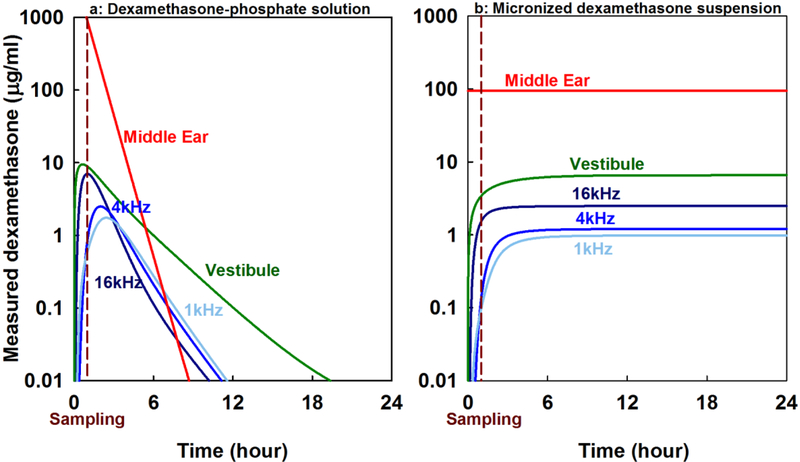
The observed rapid decline in middle ear concentration for dexamethasone-phosphate raises the question of how long it takes for perilymph dexamethasone to fall below the level achieved when dexamethasone suspension is applied. The calculated time courses of absolute (non-normalized) concentrations for the two application conditions are compared in Figure 7. Although perilymph levels are transiently higher with dexamethasone-phosphate application they rapidly decline and in just a few hours perilymph levels achieved with a dexamethasone suspension application would be higher. Dexamethasone-phosphate provides only a brief exposure to the drug for all regions of the inner ear. It should be noted that the relatively low gradients between different regions of the cochlea occur because the simulation includes the small amount of entry representing that through the thin bone at the apex of the guinea pig cochlea. Apical entry is also apparent in the 1 kHz region initially rising in concentration more rapidly in concentration than the 4 kHz region for both forms of the drug.
Figure 7:
Calculation of dexamethasone distribution as a function of location and time in the guinea pig cochlea based on pharmacokinetic parameters derived in this study. Distribution compares application of 4 mg/mL dexamethasone-phosphate solution (a) with dexamethasone suspension with a dissolved concentration of 94.2 μg/mL (b). The time at which perilymph was sampled in the current study (1 hour) is marked. Inner ear levels are shown for 3 cochlear regions, 3.5 mm (16 kHz), 8.2 mm (4 kHz) and 12.4 mm (1 kHz) from the base of scala tympani, and for the vestibule. Calculated perilymph levels are transiently higher with dexamethasone-phosphate administration (apparent in the samples collected 1 hour after application, on which these calculations are based) but subsequently rapidly decline. Even though the dexamethasone suspension has lower applied concentration perilymph levels will be higher with the suspension formulation after just a few hours.
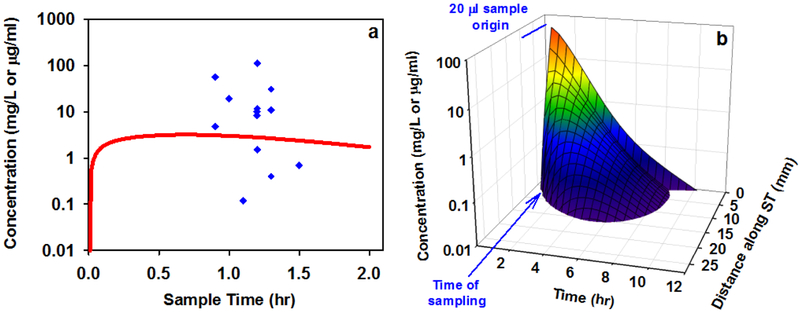
Although the potential relevance of these findings to intratympanic therapy in humans can be assessed quantitatively through computer simulations, it is important to validate whether the computer simulations accurately represent the human situation. Perilymph dexamethasone concentrations have been measured in one study in which human patients were treated with intratympanic applications of dexamethasone-phosphate [Bird et al. 2011]. A comparison between the measured concentrations and the concentration time course derived by simulation of dexamethasone-phosphate application to a human-sized ear is shown in Figure 8a. In the Bird study a 20 μL perilymph sample was aspirated though the round window membrane of each patient. In the simulation we calculated concentration for the basal 9.1 mm of scala tympani, which has a volume of 20 μL. Round window and stapes permeabilities were those given in Table 1 for a dexamethasone-phosphate solution and elimination rates from the middle ear and from perilymph were those for dexamethasone presented earlier. In the simulations, neither the applied drug volume nor the variations in the timing of sample collection had a large influence on sample concentration. The variation in measurements must be attributed to other, unknown sources. The concentrations predicted by the model provided a relatively good representation of the amount of drug found in the human, with concentrations within a factor of two of those representing a best-fit to the albeit variable data. This confirms that simulations based on the physics of diffusion and flow, combined with the physiological processes of entry from the middle ear and elimination from perilymph established in animals can provide a rational basis for predicting drug concentrations and distribution in the human ear. The calculated distribution of dexamethasone along the cochlea as a function of time for the above simulation is shown in Figure 8b. Intratympanic application of dexamethasone-phosphate solution is calculated to provide only a brief exposure of the basal half of the cochlea to the drug. The rapid kinetics result from high rates of elimination from the both the middle ear and from perilymph.
Figure 8:
(a) Comparison of the dexamethasone time course from a simulation representing the human (red line) with perilymph sample measurements from humans as reported by Bird et al., 2011 (blue symbols). The simulation used kinetic parameters derived from the guinea pig data in this study and inner ear dimensions corresponding to the human. The model prediction lies well within the scatter of measurements, validating the ability of the model with the parameters derived here to predict dexamethasone concentrations within the range of those measured in the human cochlea. (b) Distribution of dexamethasone as a function of distance and time along scala tympani from the same simulation in which the line in (a) was derived. Intratympanic dexamethasone-phosphate therapy is calculated to provide only a brief exposure of basal half of the cochlear to drug. The spatial origin and the timing of the 20 μL samples collected by Bird et al. are indicated.
4. Discussion
Many barriers of the body are comprised of sheets of epithelial or endothelial cells in which the cells are bound tightly together by tight and adherens junctions [Giepmans & van Ijzendoorn 2009]. Unless actively transported, small molecules must pass through the lipid membranes of the cell layer in order to traverse the barrier. Lipid solubility and number of polar groups on the molecule have been shown to strongly influence how readily different molecules pass through such cellular boundaries [Egan et al., 2000; Daina & Zoete, 2016]. Access of drugs to the inner ear is also largely regulated by cellular boundaries with tight junctions [Salt & Hirose, 2018]. The endothelial cells making up the vasculature of the inner are joined together by tight junctions, providing the blood-perilymph and blood-strial barriers, restricting entry from the blood to the inner ear and controlling the elimination of molecules from perilymph to blood. Likewise, the main routes of access between the middle ear cavity and perilymph, the round window membrane and the stapes, are covered on their middle ear side by the epithelium of the middle ear. These cells are joined together by tight junctions [Goycoolea 2001]. Permeability of these boundaries has been shown to be influenced by alcohols and detergents [Li et al., 2018], substances likely to act on lipid membranes. Passage of molecules across the RW membrane and stapes is therefore appears to be limited by passage though the lipid membranes of the covering middle ear epithelium.
In our prior study, we found that after loading perilymph with drug, dexamethasone-phosphate was lost considerably more slowly than dexamethasone [Salt et al., 2012]. Our current analysis also leads us to conclude that dexamethasone-phosphate similarly enters perilymph from the middle ear considerably more slowly than dexamethasone. These findings are in accordance with the calculated properties of the two molecules shown in Figure 1 in which the more polar form, dexamethasone-phosphate, will pass less easily through membranous boundaries. Unfortunately, the difference in properties between dexamethasone-phosphate (less permeable) and dexamethasone (more permeable) confer an unfortunate combination of molecular properties for the use of dexamethasone-phosphate in local drug therapy of the ear. Dexamethasone-phosphate enters perilymph relatively slowly and is then metabolized to the more permeable form, which is eliminated rapidly from perilymph. The fast rate of elimination results in short exposure times of the inner ear to drug, as shown in Figure 7.
One factor that needs to be considered in comparing guinea pig and human drug applications is whether middle ear kinetics could differ between the human and the guinea pig as a larger volume of drug is typically applied to humans (1 mL in humans compared to 20 nL in guinea pigs). A larger volume could be expected to exhibit slower kinetics. However, in our experiments drug solution continued to fill the RW niche at the end of the experiment as the animals were oriented partially inverted with the ventrolateral aspect upwards and anesthetized animals do not swallow. In humans, the head may be oriented to restrict loss down the Eustachian tube for a brief period (30 mins), but then the patient typically returns to the normal, upright posture. As they are conscious, through a combination of swallowing and the action of the ciliated epithelium of the ventral mastoid, the applied drug volume may be rapidly cleared. It is therefore difficult to know whether the decline of drug concentration will be faster or slower in the guinea pig than the human and humans could actually exhibit faster loss from the middle ear. As the retention time in the middle ear plays a major factor in the perilymph drugs level achieved, the kinetics of drugs in the human middle ear needs to be measured.
It could also be reasoned that the poor entry kinetics of dexamethasone-phosphate could be compensated by applying higher drug levels. The comparisons were made here with a commonly-used concentration of dexamethasone-phosphate of 4 mg/mL. There are now reports with higher dosing levels in humans, using up to 24 mg/mL [Alexander et al., 2015]. This would be expected to increase perilymph concentrations by about a factor of 6 (2/3 of a decade on the logarithmic scales), but the increased dosing would still be transient compared to the use of a dexamethasone suspension.
In humans, the gradients of dexamethasone concentration along the cochlea are expected to be substantially larger than shown here for the guinea pig in Figure 7. The human cochlea is approximately twice the length of the guinea pig which almost doubles the gradient when concentration is shown on a logarithmic scale. In addition, drug passage through the thin bone at the apex of the guinea pig ear [Mikulek et al., 2009] and which reduces the measured gradients measured, is unlikely to contribute in humans where the bony encasement of the inner ear is much thicker.
The perilymph dexamethasone concentrations found in the present study are generally comparable with prior animal studies, even though different application conditions, sampling methods and timing of sample collection have been used. For dexamethasone-phosphate solution we found perilymph to reach 0.13% of the applied concentration, which is less than that found in some studies [Creber et al., 2018; Plontke et al., 2008] but more than found in others [Liu et al., 2006; Wang et al., 2011]. In one study [Wang et al. (2011] perilymph levels were compared for dexamethasone-phosphate given in poloxamer gel and found to be lower with poloxamer in the formulation, similar to the result seen here. Those measurements were based on samples collected 6 hours after application compared to the 1 hour application used here. With an applied dexamethasone suspension, one prior study found lower perilymph levels than reported here [Salt et al, 2011] while another found higher levels [Wang et al., 2011]. We found similar concentrations with and without poloxamer while Wang et al. [2011] found lower levels with poloxamer-based suspension, again based on samples collected 6 hours after administration. This same study did find similar perilymph levels with 2% dexamethasone-phosphate solution applied, compared to a 1.5% dexamethasone suspension [Wang et al., 2011]. This is in good agreement with our observations in Figure 2 and with our conclusion of a slower entry of dexamethasone-phosphate, as the dissolved drug level would have been far higher in the dexamethasone-phosphate solution compared to the suspension.
We found that Poloxamer 407 in the medium did not appreciably alter the permeability of the RW membrane and stapes, as shown in Table 1. Although Poloxamer had a negligible influence on dexamethasone entry when applied as a suspension, it was found to reduce entry substantially when applied with dexamethasone-phosphate solution. This may be partially accounted for by the gel slowing the rate of metabolism of dexamethasone-phosphate to dexamethasone. At the time of middle ear sampling the remaining drug was 71% dexamethasone for the liquid solution, but only 54% when applied as a gel. We speculate that the gel formulation does not mix as readily with middle ear contents so the gel contents will be less influenced by phosphatases present on the middle ear mucosa. In the case of the gel, more dexamethasone would remain present as the less permeable, phosphate form. It should also be noted that the lower rate of entry we measured with dexamethasone-phosphate applications occurs even though some of the drug is being converted to the permeable form in an amount that will be changing with time. As a result, the actual permeability of dexamethasone-phosphate may be even slower than we have measured here. An alternative explanation is that poloxamer does affect (decrease) permeability of the entry barriers of the ear but the permeability change only influences the low-permeability form of the drug (dexamethasone-phosphate) and is insignificant for the already permeable from (dexamethasone). However, Li et al (2018) found that poloxamer gel did not alter entry of fluorescent dexamethasone, a larger and even less permeable form of the drug. There is therefore no strong case for poloxamer gel influencing the permeability of the barriers between the middle ear and perilymph.
In summary, we find that dexamethasone-phosphate, which is more polar and therefore more soluble in aqueous solution, has quite undesirable pharmacokinetic properties for local therapy of the ear. It enters the RW membrane and stapes considerably more slowly than the less polar form, dexamethasone, and within the ear is metabolized to dexamethasone, a more permeable form which is rapidly eliminated. As a result, local therapy with dexamethasone-phosphate is likely to produce only a brief exposure of basal cochlear and vestibular regions to the drug. Other drugs with more favorable pharmacokinetic properties for the ear need to be considered.
Acknowlegements
The research reported in this publication was supported by the National Institutes on Deafness and Other Communication Disorders (NIDCD) of the National Institutes of Health (NIH) under award number R01 DC001368. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Financial Disclosure: ANS is a paid consultant to Otonomy, Inc. Fabrice Piu and Jennifer Hou are employees of Otonomy, Inc. This project was not funded by Otonomy. Other research projects in Dr. Salt’s laboratory have been funded by Cochlear Corp. and Hoffmann La Roche Pharmaceuticals.
Disclosure of Funding NIH
References
- Alexander TH, Harris JP, Nguyen QT, Vorasubin N. Dose Effect of Intratympanic Dexamethasone for Idiopathic Sudden Sensorineural Hearing Loss: 24 mg/mL Is Superior to 10 mg/mL. Otol Neurotol. 2015;36:1321–1327. [DOI] [PubMed] [Google Scholar]
- Bird PA, Murray DP, Zhang M, Begg EJ. Intratympanic versus intravenous delivery of dexamethasone and dexamethasone sodium phosphate to cochlear perilymph. Otol Neurotol. 2011. 32:933–936. [DOI] [PubMed] [Google Scholar]
- Cheng T, Zhao Y, Li X, Lin F, Xu Y, Zhang X, Li Y, Wang R, Lai L. Computation of Octanol-Water Partition Coefficients by Guiding an Additive Model with Knowledge J. Chem. Inf. Model 2007, 47, 2140–2148. [DOI] [PubMed] [Google Scholar]
- Creber NJ, Eastwood HT, Hampson AJ, Tan J, O’Leary SJ. A comparison of cochlear distribution and glucocorticoid receptor activation in local and systemic dexamethasone drug delivery regimes. Hear Res. 2018. March 20. doi: 10.1016/j.heares.2018.03.018. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- Daina A, Zoete V. A BOILED-Egg To Predict Gastrointestinal Absorption and Brain Penetration of Small Molecules. ChemMedChem. 2016;11:1117–1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daina A, Michielin O, Zoete V. SwissADME: a free web tool to evaluate pharmacokinetics, drug-likeness and medicinal chemistry friendliness of small molecules. Sci Rep. 2017;7:42717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egan WJ, Merz KM Jr, Baldwin JJ. Prediction of drug absorption using multivariate statistics. J Med Chem. 2000;43:3867–7387. [DOI] [PubMed] [Google Scholar]
- Giepmans BN, van Ijzendoorn SC. Epithelial cell-cell junctions and plasma membrane domains. Biochim Biophys Acta. 2009;1788:820–831. [DOI] [PubMed] [Google Scholar]
- Goycoolea MV. Clinical aspects of round window membrane permeability under normal and pathological conditions. Acta Otolaryngol. 2001;121:437–447. [DOI] [PubMed] [Google Scholar]
- Hobson CE, Alexander TH, Harris JP Primary treatment of idiopathic sudden sensorineural hearing loss with intratympanic dexamethasone. Current Opinion in Otolaryngology and Head and Neck Surgery 2016; 24: 407–412. [DOI] [PubMed] [Google Scholar]
- Lambert PR, Carey J, Mikulec AA, LeBel C; Otonomy Ménière’s Study Group. Intratympanic Sustained-Exposure Dexamethasone Thermosensitive Gel for Symptoms of Ménière’s Disease: Randomized Phase 2b Safety and Efficacy Trial. Otol Neurotol. 2016. 37:1669–1676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W, Hartsock JJ, Dai C, Salt AN. Permeation Enhancers for Intratympanically-applied Drugs Studied Using Fluorescent Dexamethasone as a Marker. Otol Neurotol. 2018. 39:639–647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu HJ, Dong MM, Chi FL. Dexamethasone pharmacokinetics in Guinea pig inner ear perilymph. ORL J Otorhinolaryngol Relat Spec. 2006;68:93–98. [DOI] [PubMed] [Google Scholar]
- Liebau A, Pogorzelski O, Salt AN, Plontke SK. Hearing Changes After Intratympanically Applied Steroids for Primary Therapy of Sudden Hearing Loss: A Meta-analysis Using Mathematical Simulations of Drug Delivery Protocols. Otol Neurotol. 2017;38:19–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mikulec AA, Plontke SK, Hartsock JJ, Salt AN. Entry of substances into perilymph through the bone of the otic capsule after intratympanic applications in guinea pigs: implications for local drug delivery in humans. Otol Neurotol. 2009; 30:131–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mynatt R, Hale SA, Gill RM, Plontke SKR, Salt AN Demonstration of a longitudinal concentration gradient along scala tympani by sequential sampling of perilymph from the cochlear apex. J Assoc Res Otolaryngol. 2006;7:182–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piu F, Wang X, Fernandez R, Dellamary L, Harrop A, Ye Q, Sweet J, Tapp R, Dolan DF, Altschuler RA, Lichter J, LeBel C. OTO-104: a sustained-release dexamethasone hydrogel for the treatment of otic disorders. Otol Neurotol. 2011. 32:171–179. [DOI] [PubMed] [Google Scholar]
- Plontke SK, Biegner T, Kammerer B, Delabar U, Salt AN Dexamethasone concentration gradients along scala tympani after application to the round window membrane. Otology & Neurotology 2008; 29:401–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plontke SK, Löwenheim H, Mertens J, Engel C, Meisner C, Weidner A, Zimmermann R, Preyer S, Koitschev A, Zenner HP. Randomized, double blind, placebo controlled trial on the safety and efficacy of continuous intratympanic dexamethasone delivered via a round window catheter for severe to profound sudden idiopathic sensorineural hearing loss after failure of systemic therapy. Laryngoscope. 2009; 119:359–69. [DOI] [PubMed] [Google Scholar]
- Salt AN, Hartsock JJ, Plontke SK, LeBel C, Piu F. Distribution of dexamethasone and preservation of inner ear function following intratympanic delivery of a gel-based formulation. Audiology & Neurotology 2011;16:323–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salt AN, Hartsock JJ, Gill RM, Piu F, Plontke SK. Perilymph pharmacokinetics of markers and dexamethasone applied and sampled at the lateral semi-circular canal. J Assoc Res Otolaryngol. 2012; 13:771–783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salt AN, Gill RM, Hartsock JJ. Perilymph Kinetics of FITC-Dextran Reveals Homeostasis Dominated by the Cochlear Aqueduct and Cerebrospinal Fluid. J Assoc Res Otolaryngol. 2015;16:357–371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salt AN, Hirose K. Communication pathways to and from the inner ear and their contributions to drug delivery. Hear Res. 2018; 362:25–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoo DP, Tan GX, Ehrenburg MR, Pross SE, Ward BK, Carey JP. Intratympanic (IT) Therapies for Menière’s Disease: Some Consensus Among the Confusion. Curr Otorhinolaryngol Rep. 2017; 5:132–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Dellamary L, Fernandez R, Harrop A, Keithley EM, Harris JP, Ye Q, Lichter J, LeBel C, Piu F. Dose-dependent sustained release of dexamethasone in inner ear cochlear fluids using a novel local delivery approach. Audiol Neurootol. 2009;14(6):393–401. [DOI] [PubMed] [Google Scholar]
- Wang X, Dellamary L, Fernandez R, Ye Q, LeBel C, Piu F. Principles of inner ear sustained release following intratympanic administration. Laryngoscope. 2011; 121:385–91. [DOI] [PubMed] [Google Scholar]