Figure 2.
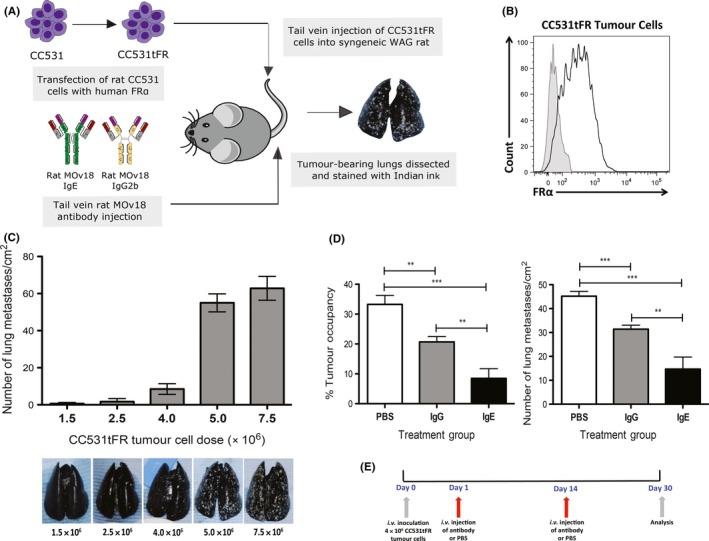
Establishment of the tumour‐bearing surrogate rat model and antibody‐mediated tumour growth restricting potencies. A, Schematic representation of immunocompetent model design: rMOv18 IgE engages native rat FcεR‐expressing immune effector cells to target syngeneic rat tumour cells expressing human folate receptor α (FRα). B, FRα expression of CC531tFR cells immediately prior to in vivo tumour challenge. C, Determination of optimum i.v. CC531tFR tumour challenge. Representative images of Indian ink‐stained lungs reveal white tumour lesions (n = 5). D, Rat MOv18 IgE demonstrates superior tumour growth restriction. Percentage (%) tumour occupancy and number of metastases/cm2 quantified following two doses (every 14 days) of PBS, or rMOv18 IgE and IgG2b at 10 mg/kg (n = 6). rMOv18 IgE compared with rMOv18 IgG2b (mean % tumour occupancy: 8.5% vs 20.7%, P = .003; mean number of lung metastases/cm2: 14.7 vs 31.4, P = .0049) or with PBS (mean % tumour occupancy: 33.3%, P < .0001; mean number of lung metastases/cm2: 45.2, P < .0001). E, Dosing regimen following tumour challenge was on days 1 and 14 over a 30‐day period [Colour figure can be viewed at wileyonlinelibrary.com]
