Figure 1.
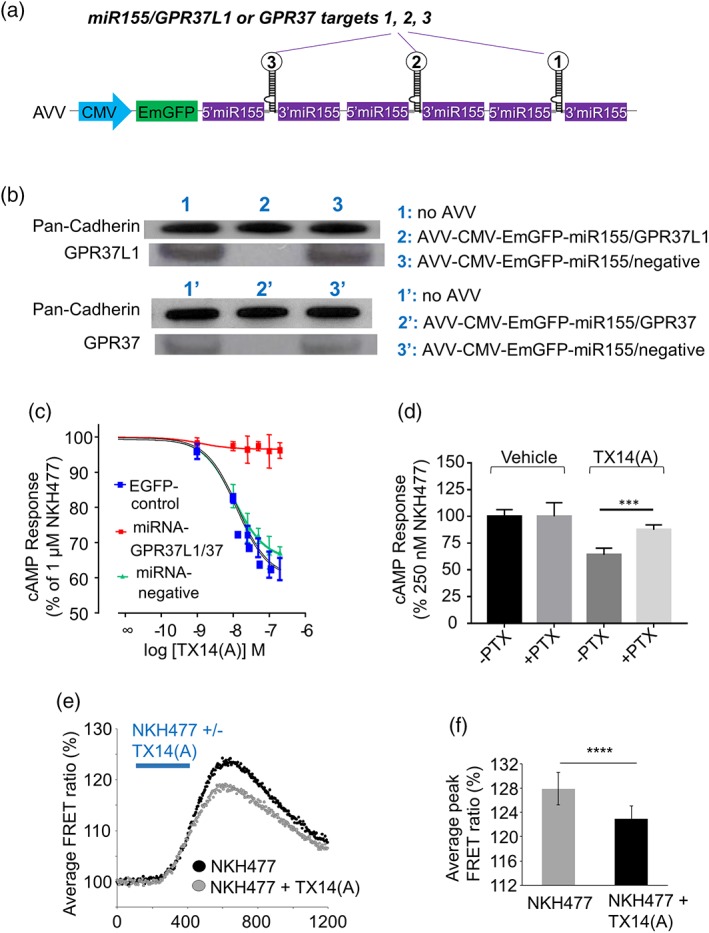
TX14(A) acting on GPR37L1/GPR37 reduces cAMP levels in astrocytes. (a) Layout of the adenoviral vectors for knock‐down of GPR37L1 and GPR37. Each vector allows co‐cistronic expression of three pre‐miRNAs targeting different regions of the target gene. AVV = human adenoviral vectors serotype 5; CMV = human cytomegalovirus promoter; EmGFP = Emerald green fluorescent protein; miR155 = flanking pre‐miRNA sequence derived from miR‐155. (b) Western blot confirms that AVV–CMV–EmGFP–miR155/GPR37L1 and AVV–CMV–EmGFP–miR155/GPR37 (MOI 10) efficiently knock‐down GPR37L1 and GPR37 in astrocytes. AVV–CMV–EmGFP–miR155/negative is a control vector with hairpin sequence relevant to no known vertebrate gene. (c) Concentration–response curves for inhibition of cAMP production by TX14(A) in astrocytes pretreated with 1 μM NKH477. Cells were transduced with AVV–CMV–Glosensor and either AVV–CMV–EmGFP (control), a mixture of AVV–CMV–EmGFP–miR155/GPR37L1 and AVV–CMV–EmGFP–miR155/GPR37 to knock‐down GPR37L1/GPR37, or with AVV–CMV–EmGFP–miR155/negative (n = 4, triplicates). (d) AVV–CMV–Glosensor transduced astrocytes were pretreated with PTX (20 hr, 100 ng/mL). About 100 nM TX14(A)‐induced cAMP reduction in astrocytes was PTX sensitive (n = 12, ***p < .001 vs. indicated group, one‐way ANOVA with Turkey's post hoc analysis). (e): Astrocytes expressing an EPAC‐based cAMP sensor were kept in PSAP‐depleted media overnight and were stimulated with NKH477 (0.5 μM) in the absence or presence of TX14(A) (100 nM). TX14(A) decreased the transient cAMP signal; average of 58 astrocytes from four experiments. (f) Pooled data from (e) shows significantly decreased FRET ratio peaks with TX14(A) (n = 58 = ****p < .0001, paired t test) [Color figure can be viewed at wileyonlinelibrary.com]
