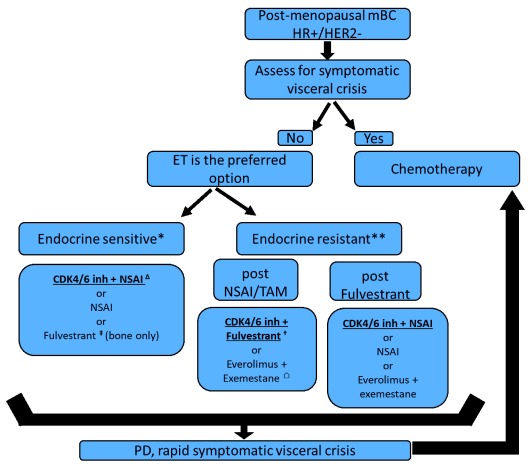
Figure 1. Current endocrine therapy in case of post-menopausal metastatic luminal breast cancer according to several pivotal trials.
∆, PALOMA-2, MONARCH-3, MONALEESA-2 trials; †, PALOMA-3, MONARCH-2, MONALEESA-3 trials; ‡, FALCON; ⌂, BOLERO-2 trial. The options printed in bold and underlined are the preferred options. For pre-menopausal women, the same algorithm may apply, with adjunction of ovarian suppression or ablation. *Endocrine-sensitive metastatic breast cancer (mBC) is defined in this algorithm as de novo luminal breast cancer or a disease that recurred more than 1 year after the end of adjuvant ET. **Endocrine-resistant mBC is defined as an mBC progressing while on ET or recurring less than 12 months after the end of adjuvant ET or during ET for metastatic disease. CDK4/6 inh, cyclin-dependent kinase 4/6 inhibitor; HER2 −, human epidermal growth factor receptor 2–negative; HR +, hormone receptor–positive; NSAI, non-steroidal aromatase inhibitor; PD, progressive disease; TAM, tamoxifen.