Abstract
Carbonic anhydrase IX (CA IX) has been identified as a biomarker and drug target for several malignant tumors due to its role in cancer cell growth and proliferation. Simple cyclic sulfonamides, like saccharin (SAC), have shown up to a 60-fold selectivity towards CA IX over other ubiquitous CA isoforms, with greater selectivity obtained applying the “tail-approach” to derivatize SAC with a methylene triazole linker that connected to a “tail” beta glucoside. These modifications of SAC led to an increased selectivity of more than 1000-fold towards CA IX, whereas clinically available CA inhibitors show little to no isoform selectivity. As part of our interest in the development of new CA inhibitors, we found the existing synthetic protocol, which relies on a N-tert-butyl saccharin intermediate, to be problematic in the final deprotection steps. We therefore describe an alternative approach to the synthesis of these compounds featuring a gentle “one pot” deprotection/cyclization as the final synthetic step, and report new galactosyl and glucosyl conjugates with low to mid nM inhibition of CA IX.
Keywords: click chemistry, benzosulfimide, saccharin, carbonic anhydrase, glucose, galactose, inhibitor
1. Introduction
Carbonic anhydrase IX (CA IX) belongs to a family of zinc metalloenzymes that catalyze the interconversion of carbon dioxide and water to bicarbonate and a proton.[1–3] These enzymes play roles in a broad range of physiological processes including pH regulation, ion transport, and signal transduction.[4, 5] There are 15 human CA isoforms expressed in specific subcellular locations including cytosolic, mitochondrial and membrane-bound forms.[6] All enzymatically active CAs contain a highly conserved active site consisting of a zinc ion coordinated by three histidine residues (H94, H96, H119, CA II numbering) and a zinc-bound solvent (ZBS, H2O/-OH).[7, 8] Due to their association with various diseases such as glaucoma, epilepsy and cancer, many of these CA isoforms have become drug targets.[9–13]
CA IX is a membrane-bound isoform with an extracellular catalytic site, and has been associated with metastatic tumors.[14, 15] With these tumor types, rapidly proliferating cancer cells outgrow the blood supply, causing a reduction in extracellular oxygen availability and acidification of the extracellular environment, also known as tumor hypoxia.[16, 17] This hallmark of metastatic cancers leads to an alteration in cell metabolism from mitochondrial oxidative phosphorylation to anaerobic glycolysis, termed the Warburg effect.[18] This phenomenon results in increased lactic acid being exported from the cell, decreasing the extracellular pH and increasing the expression of pH regulators, such as CA IX, to buffer the pH of the tumor microenvironment.[14, 15, 18] As such, inhibition of CA IX has been shown to reduce tumor cell survival and proliferation making it a good anticancer target.[19–21] Due to this association with tumor hypoxia, CA IX has also been identified as a biomarker for metastatic cancers.[22, 23]
Currently, there are over 20 clinically used CA inhibitors, but due to the highly conserved active site across the isoforms these inhibitors lack specificity. There is a need to develop isoform-selective inhibitors that target CA IX as potential anticancer chemotherapeutics.[24] Structural differences between the isoforms have been exploited to develop more isoform-specific inhibitors.[10, 25, 26] For example, CA IX has a wider active site entrance when compared to CA II, a ubiquitously expressed isoform, due to a residue variation of valine in CA IX and phenylalanine in CA II at the 131 position. This difference allows bulkier inhibitors to bind within the CA IX active site. Also, differing residues ~15 Å from the catalytic zinc have been identified, and define a region known as the “selective pocket”.[27] One of the most successful approaches to target this region has been the “tail-approach” which includes a zinc binding group (ZBG) to anchor the compound to the catalytic zinc, a linker region to extend the compound out of the active site and a tail moiety to interact with unique residues within the selective pocket.[28–31]
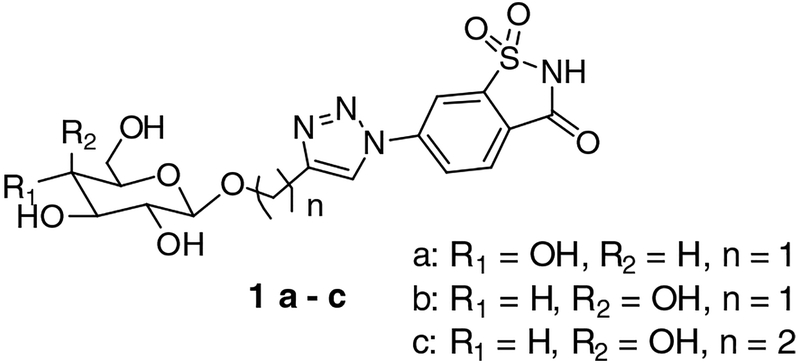
Previous studies have shown that certain artificial sweeteners bind selectively to CA IX, some with nanomolar affinities.[32–35] The artificial sweetener saccharin has been used as a ZBG in drug development using the “tail-approach”[36–37] along with a methylene triazole linker and glucose tail moiety (compound 1a, Figure 1).[38] The saccharin-based compound (SBC) displayed over 1000-fold selectivity for CA IX over off-target CA isoforms CA I and CA II.[38] This increased specificity could be attributed to interactions made by the 2’-OH of the glucose moiety with Gln67 and Gln92, as well as an added hydrophobic interaction with an O-methylene of the tail sugar and Leu91, which is hypothesized to aid in selectivity for CA IX.[26, 27]
Figure 1.
Structures of saccharin-glycoconjugates
The high selectivity displayed by the saccharin-glycoconjugate 1a[39] led us to the design of new derivatives characterized by the presence of a saccharin moiety connected to a sugar with an intervening triazole spacer, assembled by a Cu-catalyzed click reaction.[40–44] We focused our attention on modifications affecting the nature of the sugar scaffold by replacing β-glucose with β-galactose together with increasing the length of the triazole spacer by introducing a two-carbon ethyl linker between the glyosidic oxygen and the triazole ring (compounds 1b and 1c, Figure 1). With these modifications, our idea was to control the binding interactions of the linker and tail region of the saccharin-sugar compound with CA IX residues.
2. Materials and Methods.
2.1. Chemistry.
All reagents for chemical synthesis were purchased from Fisher Scientific (Pittsburg, PA) and Sigma-Aldrich (St. Louis, MO). Trifluoroacetic acid (TFA) was freshly distilled from phosphorous pentoxide prior to use. Unless otherwise specified, all reactions were carried out in anhydrous conditions and under an inert atmosphere of argon or nitrogen. Reactions were monitored by thin-layer chromatography (TLC) on glass plates precoated with silica gel 60 (F-254, EMD Millipore), and visualized with UV light at λ = 254 nm. Glassware was flame dried or oven dried prior to use. The synthesized compounds were purified by flash chromatography on silica gel (230–400 mesh particle size, pore size 60 Å, Sigma-Aldrich). Melting points were obtained on an MFB-595010M Gallenkamp apparatus equipped with a digital thermometer. NMR Spectra (1H and 13C) were recorded on a Varian Mercury-300 (300 and 75.0 MHz) instrument using CDCl3, (CD3)2CO, (CD3)2SO or D2O as solvent at 20 °C. Chemical shifts are reported in parts per million (ppm) relative to the peak internal TMS standard (δ = 0.00 ppm) for CDCl3, CD3OD, (CD3)2CO and (CD3)2SO and relative to the peak of the solvent for D2O in 1H NMR spectra and relative to the central peak of the solvent (δ = 77.16 ppm for CDCl3, 29.84 ppm for (CD3)2CO and 39.52 ppm for (CD3)2SO) in 13C NMR spectra. Abbreviations used for peak multiplicities in 1H NMR are: app, apparent; s, singlet; br s, broad singlet; d, doublet; t, triplet; m, multiplet; dd, doublet of doublets; dt, doublet of triplets; td, triplet of doublets; ddd, doublet of doublet of doublets. Processing of the spectra was performed with MestReNova 8.1.1. Mass spectra were obtained on a Hewlett-Packard 5988A spectrometer or on an Agilent 6220 ESI TOF (Santa Clara, CA) mass spectrometer equipped with electrospray and DART sources operated in positive ion mode. The mass spectroscopic data analyses were performed by the UF Chemistry Department (Funding from NIH S10 OD021758-01A1).
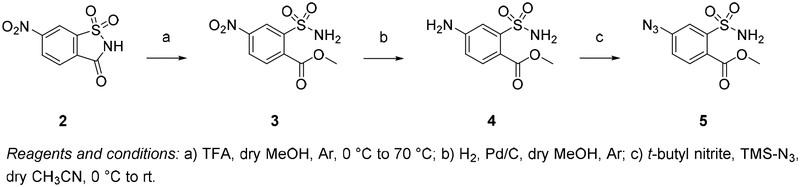
Methyl 4-nitro-2-sulfamoylbenzoate (3).
Following is a variation of the previously reported method.[45] In an oven dried round bottom flask flushed with argon, nitro-saccharin (2) (1 g, 4.38 mmol, 1 equiv) was dissolved in 50 mL dry MeOH, cooled to 0 °C and 0.5 mL of dry TFA was added dropwise. The ice-bath was then removed, and the reaction mixture was allowed to warm to rt before refluxing it at 70 °C for 18 hours. Upon no further reaction progress (TLC in hexane/EtOAc 1:1), the reaction mixture was cooled to rt and the solvent was evaporated under reduced pressure. The resulting crude was separated from unreacted starting material by recrystallization from methanol. White crystals (511 mg, 45%). mp 191–193 °C. 1H NMR (300 MHz, (CD3)2SO) δ 8.74 (d, J = 2.3 Hz, 1H), 8.50 (dd, J = 8.5, 2.3 Hz, 1H), 7.94 (d, J = 8.4 Hz, 1H), 7.76 (br s, 2H), 3.88 (s, 3H); 13C NMR (75 MHz, (CD3)2SO) δ 166.4, 148.3, 142.9, 136.1, 130.6, 126.8, 122.6, 53.4; HRMS (ESI) of C8H8N2O6S [39]: calcd for [M + Na]+ 283.0001, found 283.0007.
Methyl 4-amino-2-sulfamoylbenzoate (4).
[45] Under anhydrous and inert argon atmosphere, methyl-4-nitro-2-sulfamoylbenzoate (3) (716 mg, 2.75 mmol, 1 equiv) was dissolved in 50 mL dry MeOH and hydrogenated at 1 atm over 10 % Pd/C (50 mg) at rt. The reaction was complete by TLC after 1 h (hexane/EtOAc 3:7). The reaction mixture was filtered through a Celite pad, which was then washed with MeOH. Evaporation of the solvent under reduced pressure quantitatively afforded the desired compound 4, used without further purification. Light yellow solid (627 mg, yield 99 %). 1H NMR (300 MHz, (CD3)2CO) δ 7.72 (d, J = 8.5 Hz, 1H), 7.39 (d, J = 2.4 Hz, 1H), 6.83 (dd, J = 8.5, 2.4 Hz, 1H), 6.63 (br s, 2H), 5.84 (br s, 2H), 3.85 (s, 3H); 13C NMR (75 MHz, (CD3)2CO) δ 168.3, 153.3, 145.8, 134.5, 116.0, 115.5, 114.4, 52.7; HRMS (ESI) of C8H10N2O4S [M]: calcd for [M + Na]+ 253.0253, found 253.0261.
Methyl 4-azido-2-sulfamoylbenzoate (5).
Methyl 4-amino-2-sulfamoylbenzoate (4) (250 mg, 1.09 mmol, 1 equiv) was dissolved in dry CH3CN (3 mL) and cooled to 0 °C. Tert-butyl nitrite (194 μl, 1.63 mmol, 1.5 equiv) and trimethylsilyl azide (TMS-N3) (172 μl, 1.30 mmol, 1.2 equiv) were subsequently added dropwise at 0 °C. The resulting mixture was stirred at 0 °C for 1 hour, then let warm to room temperature for 14 hours, at which time TLC showed complete consumption of the starting material (hexane/EtOAc 2:1). The solvent was evaporated under vacuum and the crude product was purified on a silica gel column (hexane/EtOAc 2:1 to 1:1) to afford the pure compound as a light yellow solid (259 mg, yield 93%). IR (solid sample, ATR cell) 2126 cm−1 (strong) ArN3; 1H NMR (300 MHz, CD3OD) δ 7.87 (d, J = 8.3 Hz, 1H), 7.71 (d, J = 2.4 Hz, 1H), 7.37 (dd, J = 8.3, 2.4 Hz, 1H), 3.94 (s, 3H); 13C NMR (75 MHz, CDCl3) δ 167.0, 144.8, 144.1, 133.2, 125.3, 122.1, 119.5, 53.6; mp 137.5–138.3 °C. HRMS (ESI) of C8H8N4O4S [M]: calcd for [M + Na]+ 279.0158, found 279.0167.
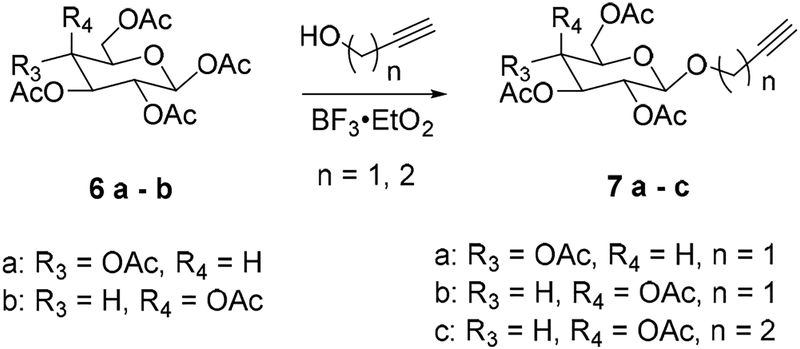
2-Propynyl 2,3,4,6-tetra-O-acetyl-β-D-glucopyranoside (7a).
[46] 3Å molecular sieves (1 g) were placed in an oven dried round bottom flask flushed with argon. β-D-glucose pentaacetate (5a) (1 g, 2.56 mmol, 1 equiv) was added and dissolved in 18 mL dry CH2Cl2, followed by addition of propargyl alcohol (298 μL, 5.12 mmol, 2 equiv). The resulting mixture was cooled to 0 °C and BF3·Et2O (2.53 mL, 20.5 mmol, 8 equiv) was added dropwise. After 16 hours at 40 °C the TLC (hexane/EtOAc 3:2) showed complete consumption of the starting material. The reaction mixture was diluted with 30 mL CH2Cl2, quenched by addition of solid NaHCO3 (3 g) and stirred at room temperature for 20 minutes. Molecular sieves were filtered off and the solution was subsequently washed with 10 % aq. NaHCO3 (x 1). The aqueous bicarbonate solution was back-extracted with CH2Cl2 and the combined organic phases were washed with brine (x 1). The organic phase was dried over anhydrous Na2SO4, filtered and concentrated under vacuum. The crude product was purified by silica chromatography eluting with a step gradient of hexane/EtOAc 4:1 to 1:1. A white solid (590 mg, yield 60 %) was obtained after removal of solvents. 1H NMR (300 MHz, CDCl3) δ 5.25 (app t, J = 9.4 Hz, 1H), 5.10 (app t, J = 9.6 Hz, 1H), 5.02 (dd, J = 9.4, 8.0 Hz, 1H), 4.78 (d, J = 7.9 Hz, 1H), 4.38 (d, J = 2.3 Hz, 2H), 4.32 – 4.24 (m, 1H), 4.15 (dd, J = 12.3, 2.3 Hz, 1H), 3.73 (ddd, J = 9.9, 4.5, 2.4 Hz, 1H), 2.47 (t, J = 2.3 Hz, 1H), 2.09 (s, 3H), 2.06 (s, 3H), 2.03 (s, 3H), 2.01 (s, 3H) 1H NMR agreed with the reported data [46]. HRMS (ESI) of C17H22O10 [M]: calcd for [M+ NH4]+ 404.1551, found 404.1562.
2-Propynyl 2,3,4,6-tetra-O-acetyl-β-D-galactopyranoside (7b).
3Å molecular sieves (1 g) were placed in an oven dried round bottom flask flushed with argon. β-D-galactose pentaacetate (5b) (1.5 g, 3.84 mmol, 1 equiv) was added and dissolved in 27 mL dry CH2Cl2, followed by addition of propargyl alcohol (447 μL, 7.68 mmol, 2 equiv). The resulting mixture was cooled to 0 °C and BF3·Et2O (3.79 mL, 30.7 mmol, 8 equiv) was added dropwise. After 16 hours at rt, TLC (hexane/EtOAc 1:1) showed complete consumption of the starting material. The reaction mixture was diluted with 45 mL CH2Cl2, then quenched by addition of solid NaHCO3 (4.5 g) followed by stirring at room temperature for 20 minutes. Molecular sieves were filtered off and the solution was subsequently washed with 10% aq. sol. NaHCO3 (x 1) and brine (x 1). The organic phase was dried over anhydrous Na2SO4, filtered and concentrated under vacuum. The crude product was purified by silica gel column chromatography, eluting in hexane/EtOAc 1:1. After removal of solvents, a syrup was obtained which solidified upon standing (1.17 g, yield 79 %). 1H NMR (300 MHz, CDCl3) δ 5.43 – 5.37 (m, 1H), 5.22 (dd, J = 10.2, 8.2 Hz, 1H), 5.10 – 5.02 (m, 1H), 4.74 (d, J = 7.9 Hz, 1H), 4.41 – 4.36 (m, 2H), 4.24 – 4.08 (m, 2H), 3.94 (t, J = 6.6 Hz, 1H), 2.47 (app s, 1H), 2.15 (s, 3H), 2.07 (s, 3H), 2.05 (s, 3H), 1.99 (s, 3H) (1H NMR matched literature reported[47] spectra); HRMS (ESI) of C17H22O10 [M]: calcd for [M + NH4]+ 404.1551, found 404.1571; calcd for [M + Na]+ 409.1105, found 409.1115.
3-butynyl 2,3,4,6-tetra-O-acetyl-β-D-galactopyranoside (7c).
[48] 3Å molecular sieves (1 g) were placed in an oven dried round bottom flask flushed with argon. β-D-galactose pentaacetate (5b) (1 g, 2.56 mmol, 1 equiv) was added and dissolved in 18 ml dry CH2Cl2, followed by addition of 3-butyn-1-ol (388 μL, 5.12 mmol, 2 equiv). The resulting mixture was cooled to 0 °C and BF3·Et2O (2.53 mL, 20.5 mmol, 8 equiv) was added dropwise. After 12 hours at rt the TLC (hexane/EtOAc 1:1) showed complete consumption of the starting material. The reaction mixture was diluted with 30 mL CH2Cl2, quenched by addition of solid NaHCO3 (3 g) and stirred at room temperature for 20 minutes. Molecular sieves were filtered off and the solution was subsequently washed with 10 % aq. NaHCO3 (x 1) and brine (x 1). The organic phase was dried over anhydrous Na2SO4, filtered and concentrated under vacuum. The crude product was purified by silica gel column chromatography, eluting with a step gradient of hexane/EtOAc 2:1 to 1:1 to afford the product as a colorless solid. (681 mg, 66 % yield). 1H NMR (300 MHz, CDCl3) δ 5.39 (d, J = 3.4 Hz, 1H), 5.21 (dd, J = 10.5, 7.9 Hz, 1H), 5.03 (dd, J = 10.5, 3.4 Hz, 1H), 4.55 (d, J = 7.9 Hz, 1H), 4.23 – 4.08 (m, 2H), 4.00 – 3.89 (m, 2H), 3.68 (dt, J = 9.8, 7.3 Hz, 1H), 2.48 (td, J = 6.8, 2.5 Hz, 2H), 2.15 (s, 3H), 2.07 (s, 3H), 2.06 (s, 3H), 2.01 – 1.96 (m, 4H); 13C NMR (75 MHz, CDCl3) δ 170.4, 170.3, 170.2, 169.5, 101.4, 80.7, 70.9, 70.8, 69.6, 68.7, 67.9, 67.1, 61.3, 20.9, 20.7, 20.7, 20.6, 19.9 (1H and 13CNMR matched literature reported spectra)[48, 49]; mp 89.8 – 90.2 °C. HRMS (ESI) of C18H24O10 [M]: calcd for [M + NH4]+ 418.1708, found 418.1695.
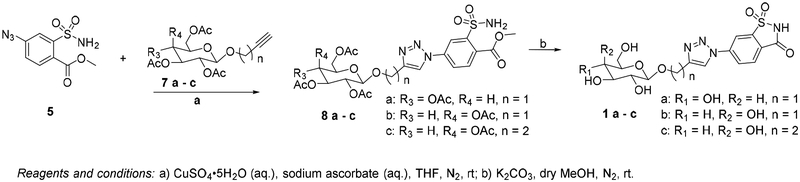
{1-[4-(methoxycarbonyl)-3-sulfamoylphenyl]-1H-1,2,3-triazol-4-yl} methyl 2,3,4,6-tetra-O-acetyl-β-D-glucopyranoside (8a).
In a round bottom flask under nitrogen, 7a (90 mg, 0.23 mmol, 1 equiv) was dissolved in 2.2 mL THF. Azide 5 (60 mg, 0.23 mmol, 1 equiv) was added to the stirred suspension, followed by 0.1 M aq. sodium ascorbate (19 mg, 0.09 mmol, 0.4 equiv) and 0.1 M aq. CuSO4·5H2O (12 mg, 0.05 mmol, 0.2 equiv). The resulting suspension was stirred at rt for 13 hours. Upon completion (TLC in CH2Cl2/MeOH 9:1) the reaction mixture was diluted with H2O and extracted with CH2Cl2 (x 2). The combined organic phases were dried over anhydrous Na2SO4, filtered and concentrated under vacuum. The crude was purified by silica gel column chromatography (CH2Cl2/MeOH 98:2). The product was obtained as a white solid (142 mg, 95 %). 1H NMR (300 MHz, CDCl3) δ 8.45 (d, J = 2.1 Hz, 1H), 8.19 – 8.13 (m, 2H), 8.07 (d, J = 8.4 Hz, 1H), 5.92 (s, 2H), 5.23 (app t, J = 9.5 Hz, 1H), 5.17 – 4.91 (m, 4H), 4.73 (d, J = 7.9 Hz, 1H), 4.24 (dd, J = 12.3, 4.6 Hz, 1H), 4.17 (dd, J = 12.1, 1.9 Hz, 1H), 4.04 (s, 3H), 3.79 – 3.71 (m, 1H), 2.08 (s, 3H), 2.03 (s, 6H), 2.01 (s, 3H); 13C NMR (75 MHz, CDCl3) δ 170.9, 170.2, 169.6, 169.5, 166.8, 145.8, 144.0, 138.9, 132.9, 129.3, 123.1, 121.4, 119.9, 100.3, 72.7, 72.0, 71.3, 68.3, 62.9, 61.9, 53.8, 20.8, 20.7, 20.6 (2C); mp 82.6–85.4 °C; HRMS (ESI) of C25H30N4O14S [M]: calcd for [M + H]+ 643.1552, found 643.1559; calcd for [M +Na]+ 665.1371, found 665.1376.
{1-[4-(methoxycarbonyl)-3-sulfamoylphenyl]-1H-1,2,3-triazol-4-yl} methyl 2,3,4,6-tetra-O-acetyl-β-D-galactopyranoside (8b).
In a round bottom flask under nitrogen, 7b (75 mg, 0.19 mmol, 1 equiv) was dissolved in 1.8 mL THF. Azide 5 (50 mg, 0.19 mmol, 1 equiv) was added to the stirred suspension, followed by 0.1 M aq. sol. sodium ascorbate (16 mg, 0.08 mmol, equiv) and 0.1 M aq. sol. CuSO4·5H2O (10 mg, 0.04 mmol, 0.2 equiv). The resulting suspension was stirred at rt for 16 hours. Upon completion (TLC in CH2Cl2/MeOH 9:1) the reaction mixture was diluted with H2O and extracted with CH2Cl2 (x 2). The organic phases were combined, dried over anhydrous Na2SO4, filtered and concentrated under vacuum. The crude product was purified by silica gel column chromatography, eluting in a step gradient with hexane/EtOAc 1:1 to 1:3 The product was obtained as a colorless oil (104 mg, yield 76%). 1H NMR (300 MHz, CDCl3) δ 8.42 (app s, 1H), 8.17 (s, 1H), 8.13 (app d, J = 8.4 Hz, 1H), 8.06 (d, J = 8.3 Hz, 1H), 6.05 2H), 5.42 (d, J = 2.5 Hz, 1H), 5.26 (dd, J = 10.2, 8.1 Hz, 1H), 5.09 – 5.01 (m, 2H), 4.93 (d, J 13.0 Hz, 1H), 4.71 (d, J = 7.8 Hz, 1H), 4.24 – 4.07 (m, 2H), 4.03 (s, 3H), 3.98 (t, J = 6.6 1H), 2.17 (s, 3H), 2.03 (s, 6H), 1.99 (s, 3H); 13C NMR (75 MHz, CDCl3) δ 170.6, 170.3, 170.2, 169.8, 166.8, 145.9, 144.0, 138.9, 132.9, 129.3, 123.1, 121.3, 119.8, 100.7, 71.0, 70.8, 68.8, 67.1, 62.8, 61.4, 53.8, 20.9, 20.8, 20.7, 20.6. HRMS (ESI) of C25H30N4O14S [M]: calcd for [M + H]+ 643.1552, found 643.1539; calcd for [M + Na]+ 665.1371, found 665.1363.
{1-[4-(methoxycarbonyl)-3-sulfamoylphenyl]-1H-1,2,3-triazol-4-yl} ethyl 2,3,4,6-tetra-O-acetyl-β-D-galactopyranoside (8c).
In an oven dried round bottom flask under nitrogen, 7c (96 mg, 0.24 mmol, 1 equiv) was dissolved in 1.5 mL dry THF. Azide 5 (62 mg, 0.24 mmol, 1 equiv) was added to the stirring suspension, followed by 0.1 M aq. sol. sodium ascorbate (20 mg, 0.10 mmol, 0.4 equiv) and 0.1 M aq. sol. CuSO4·5H2O (12 mg, 0.05 mmol, 0.2 equiv). The resulting suspension was heated at 40 °C for 20 hours. Upon completion (TLC in CH2Cl2/MeOH 9:1) the reaction mixture was cooled to rt and extracted with CH2Cl2 (x 2). The organic phases were combined, dried over anhydrous Na2SO4, filtered and concentrated under vacuum to afford the pure desired compound as a yellow oil (123 mg, yield 78%). 1H NMR (300 MHz, CDCl3) δ 8.49 (d, J = 2.2 Hz, 1H), 8.25 (dd, J = 8.6, 2.1 Hz, 1H), 8.17 (s, 1H), 8.06 (d, J = 8.4 Hz, 1H), 6.00 (s, 2H), 5.42 (d, J = 2.3 Hz, 1H), 5.32 – 5.20 (m, 1H), 5.02 (dd, J = 10.5, 3.0 Hz, 1H), 4.52 (d, J = 8.0 Hz, 1H), 4.28 – 4.07 (m, 3H), 4.03 (s, 3H), 3.99 – 3.87 (m, 2H), 3.14 (t, J = 5.8 Hz, 2H), 2.17 (s, 3H), 2.02 (s, 3H), 1.99 (s, 3H), 1.93 (s, 3H); 13C NMR (75 MHz, CDCl3) δ 170.6, 170.3, 170.1, 169.8, 166.8, 146.3, 144.0, 139.1, 132.7, 128.9, 122.6, 120.9, 119.4, 101.2, 70.7, 70.7, 68.9, 68.3, 67.0, 61.4, 53.6, 26.3, 20.7 (3C), 20.5. HRMS (ESI) of C26H32N4O14S [M]: calcd for [M + H]+ 657.1708, found 657.1724; calcd for [M +Na]+ 679.1528, found 679.1545.
{1-[4-(methoxycarbonyl)-3-sulfamoylphenyl]-1H-1,2,3-triazol-4-yl} methyl-β-D-glucopyranoside (1a).
[39] In an oven dried round bottom flask under nitrogen, cycloaddition product 8a (50 mg, 0.078 mmol, 1 equiv), 6 mL dry MeOH and crushed K2CO3 (125 mg, 0.9 mmol) were added. The resulting suspension was stirred at room temperature for 2.5 h. Upon completion (TLC in CH2Cl2/MeOH 3:2), the crude product was filtered through a neutral alumina column, eluting with 3:2 MeOH/H2O. A silica gel column was applied to remove the remaining salt, eluting with a step gradient of 4:1 CH2Cl2/MeOH to 3:2 CH2Cl2/MeOH. Finally, an aqueous solution of 1a was filtered through a small Dowex 50Wx8 cation exchange column (H+ form) for further desalting. The product 1a was obtained as an amorphous white solid, mp 190–193 °C (d) (25.1 mg, yield 73 %). 1H NMR (300 MHz, D2O) δ 8.62 (s, 1H), 8.33 (app s, 1H), 8.15 (app d, J = 8.3 Hz, 1H), 7.97 (d, J = 8.2 Hz, 1H), 5.08 (d, J = 13.0 Hz, 1H), 4.97 (d, J = 12.7 Hz, 1H), 4.62 (d, J = 7.8 Hz, 1H), 3.93 (d, J = 12.0 Hz, 1H), 3.73 (dd, J = 12.0, 5.6 Hz, 1H), 3.55 – 3.46 (m, 2H), 3.41 (d, J = 9.1 Hz, 1H), 3.33 (t, J = 8.7 Hz, 1H); 13C NMR (75 MHz, D2O) δ 167.7, 144.7, 142.5, 139.9, 130.8, 125.7, 125.7, 123.6, 112.8, 101.5, 75.9, 75.6, 73.0, 69.6, 61.8, 60.7 (1H and 13CNMR agreed with literature reported spectra)[39]; HRMS (ESI) of C16H18N4O9S [M]: calcd for [M + H]+ 443.0867, found 443.0871; calcd for [M + Na]+ 465.0687, found 465.0693.
{1-[4-(methoxycarbonyl)-3-sulfamoylphenyl]-1H-1,2,3-triazol-4-yl} methyl-β-D-galactopyranoside (1b).
In an oven dried round bottom flask under nitrogen, cycloaddition product 8b (34.5 mg, 0.054 mmol, 1 equiv), 3 mL dry MeOH and crushed K2CO3 (58.8 mg, 0.43 mmol) were added. The resulting suspension was stirred at room temperature for 2.5 hours. Upon completion (TLC in CH2Cl2/MeOH 3:2), the crude product was filtered through a neutral alumina column eluting in MeOH/H2O 3:2. A silica gel column was applied to remove the remaining salt eluting with a step gradient of 7:3 CH2Cl2/MeOH to 3:2 CH2Cl2/MeOH. The product was obtained as a translucent white glass (20.1 mg, yield 85%). 1H NMR (300 MHz, D2O) δ 8.59 (s, 1H), 8.21 (app s, 1H), 8.05 (dd, J = 8.0, 1.7 Hz, 1H), 7.84 (d, J = 8.3 Hz, 1H), 5.10 (d, J = 12.8 Hz, 1H), 4.98 (d, J = 12.9 Hz, 1H), 4.57 (d, J = 7.7 Hz, 1H), 3.96 (d, J = 3.2 Hz, 1H), 3.88 – 3.73 (m, 3H), 3.69 (dd, J = 9.9, 3.3 Hz, 1H), 3.59 (dd, J = 9.9, 7.7 Hz, 1H); 13C NMR (75 MHz, D2O) δ 170.6, 144.6, 143.8, 139.3, 132.4, 125.3, 125.2, 123.6, 112.5, 102.0, 75.2, 72.7, 70.6, 68.6, 61.7, 60.9. HRMS (ESI) of C16H18N4O9S [M]: calcd for [M + H]+ 443.0867, found 443.0874; calcd for [M + Na]+ 465.0687, found 465.0691.
{1-[4-(methoxycarbonyl)-3-sulfamoylphenyl]-1H-1,2,3-triazol-4-yl} ethyl-β-D-galactopyranoside (1c).
In an oven dried round bottom flask under argon, cycloaddition product 8c (181 mg, 0.276 mmol, 1 equiv) was dissolved in 5 mL dry MeOH and crushed K2CO3 (50 mg) was added. The resulting suspension was stirred at room temperature for 40 minutes. Upon completion (TLC in CH2Cl2/MeOH 1:1), the crude product was filtered through a neutral alumina column eluting in MeOH/H2O 3:2. The K salt of the product was obtained as a colorless oil (104 mg, yield 76 %). 1H NMR (300 MHz, D2O) δ 8.35 (s, 1H), 8.15 (app s, 1H), 7.99 (app d, J = 8.1 Hz, 1H), 7.81 (d, J = 8.3 Hz, 1H), 4.48 (d, J = 7.8 Hz, 1H), 4.31 – 4.19 (m, 1H), 4.08 – 3.97 (m, 1H), 3.94 (d, J = 2.8 Hz, 1H), 3.86 – 3.63 (m, 4H), 3.54 (dd, J = 9.8, 8.1 Hz, 1H), 3.12 (t, J = 6.4 Hz, 2H); 13C NMR (75 MHz, D2O) δ 170.1, 145.9, 143.6, 138.7, 131.8, 124.8, 124.0, 121.4, 111.3, 102.9, 75.1, 72.8, 70.7, 68.7, 68.3, 60.9, 25.3. HRMS (ESI) of C17H20N4O9S [M]: calcd for [M + H]+ 457.1024, found 457.1016; calcd for [M + Na]+ 479.0843, found 479.0836; calcd for [2M + H]+ 913.1975, found 913.1960;calcd for [2M + NH4]+ 930.2240, found 930.2224; calcd for [2M + Na]+ 935.1794, found 935.1774.
2.2. Inhibition Assays.
The inhibitory activity of compounds 1a–c were estimated by their ability to inhibit the soluble form of CA IX (0.1mg/mL) and CA II (0.1mg/mL)[50] via monitoring hydrolysis of 0.8 mM p-nitrophenyl acetate in assay buffer (50mM Tris-HCl, pH 7.8) at 37 °C. The concentrations of 1a–c were varied to produce 50 % inhibition and the reported values represent an IC50. Inhibition assays with CA II used 2 μM concentrations of 1a–c, and showed no inhibition.
3. Results and Discussion
3.1. Chemistry.
The key assembly step for the synthesis of the saccharin-sugar derivatives involved the reported [39] copper mediated click reaction between a propargyl glucoside and 6-azido saccharin protected on the lactam nitrogen with a tert-butyl group. Given the acidity of the sulfimide NH group, protection was considered important to avoid interference with the cycloaddition reaction via coordination of copper[51] with the electron rich benzosulfimidate. In our studies which involved multiple attempts and variations, we were unable to satisfactorily remove the tert-butyl protecting group in refluxing TFA under the reported conditions[25]; we typically observed considerable decomposition resulting in complex mixtures. This led us to design an alternative synthetic approach, featuring a protection/deprotection strategy that was mild. We hypothesized that the ring opened version of saccharin with a sulfonamide group would be compatible with the cycloaddition chemistry, and provide entry back to the saccharin ring system after alkaline cyclization. Here we report new saccharin-glycoconjugates, obtained in good yields, under mild conditions that feature simultaneous alkaline cyclization to the saccharin ring system and sugar deprotection.
We considered saccharin derivative 5 suitable for click reaction with different peracetylated alkynyl glycosides. (Schemes 1 and 2) The desired key intermediate azide 5 was obtained in a 3 step synthesis from the commercially available nitro-saccharin (Scheme 1). First, nitro-saccharin was refluxed with TFA in methanol to promote solvolytic ring opening[52] and form 3 in 45 % yield. Compound 3 was then converted into the corresponding amino derivative 4 by catalytic hydrogenation in presence of palladium on carbon (99 % yield). The amino group of 4 was smoothly converted into the azide by reacting it with tert-butyl nitrite and trimethylsilyl azide to achieve compound 5 in 93 % yield. The syntheses of the alkynyl sugar scaffolds 7 are depicted in Scheme 2. The readily available β-D-galactose or β-D-glucose pentaacetates 6a,b were reacted with propargylic alcohol or 3-butyn-1-ol in the presence of BF3 etherate and 3Å molecular sieves to afford 7a–c in 60–79% yields after purification (Scheme 2). The copper(I)-catalyzed cycloaddition of compound 5 and alkynes 7a–c afforded the protected triazoles 8a–c in 76–95% yields (Scheme 3). These results indicate the free sulfonamide and methyl ester groups are indeed compatible with the cycloaddition chemistry, obviating the need for the tert-butyl protecting group. With the molecule assembled it remained to close the saccharin ring and deprotect the sugar. We suspected that reaction of adjacent sulfonamide and ester groups to form the saccharin ring system would be facile in alkaline conditions,[53, 54] suggesting that cyclization and methanolysis of the sugar acetyl protecting group removal could be combined into a single one-pot reaction.
Scheme 1.
Synthesis of the key intermediate methyl 4-azido-2-sulfamoylbenzoate 5.
Scheme 2.
Synthesis of acetylenic glycosides.
Scheme 3.
Cycloaddition and cyclization/deprotection sequence for saccharin-glycoconjugates.
We were pleased to find that simultaneous cleavage of the carbohydrate acetyl protecting groups and cyclization of the saccharin ring to the final compounds 1a–c was achieved in very good yields (73–85%) in a one pot reaction between 8a–c and catalytic amounts of potassium methoxide generated in situ from K2CO3 and methanol (Scheme 3).
3.2. Enzymology
Inhibition assay data are presented in Table 1. The new compound 1b is a galactose analog of the known glucosyl compound 1a, and they share similar inhibition of CA IX and a selectivity ratio of >10 for CA IX over CA II. While their IC50 values are about 8 times higher than acetazolamide (AZM), their selectivity for CA IX is superior. It is interesting that the gluco and galacto-compounds are equivalent, and this suggests that the recognition of the sugar is not dependent on the epimeric configuration of the C4 hydroxyl group. Compound 1c utilized a linker between head saccharin and tail galactose groups that was one methylene unit longer than for compounds 1a,b. This modification resulted in enhanced inhibition, halving the IC50 and doubling the selectivity ratio for CA IX over CA II. The results suggest that the longer linker has allowed better interaction of the sugar with the selectivity pocket, despite the greater flexibility anticipated for a longer linker.
Table 1.
Inhibition and selectivity ratio for CA II and CA IX with 1a-c, and AZM
| IC50 (nM) | selectivity ratioa | ||
|---|---|---|---|
| Compound | CA II | CA IX | CA II:CA IX |
| 1a | >2000 | 197 | >10 |
| 1b | >2000 | 206 | >10 |
| 1c | >2000 | 93 | >22 |
| AZMb | 12 | 25 | 0.5 |
Selectivity is determined by the ratio of IC50s for the cytosolic CA II relative to the targeted CA IX isoform.
AZM = acetazolamide
3.3. Conclusions.
We have demonstrated that saccharin nitrogen protection with the tert-butyl group can be successfully avoided, and report a useful and mild route to glucosyl and galactosyl saccharin conjugates. The new compounds display selective nM inhibition of CA IX, while not inhibiting CA II (an off-target CA) up to 2 μM. These compounds show increased isoform specificity with at least 22-fold selectivity for CA IX compared to the classically used CA inhibitor acetazolamide (AZM) which does not display selective inhibition for CA IX over CA II. These characteristics help develop the rules for a SAR for CA IX inhibitors with glycosyl tails.
Supplementary Material
Murray et al Highlights file.
An alternate and mild protection/deprotection scheme for saccharin glycoconjugates synthesis.
Linker length and sugar identity are varied.
Nanomolar inhibition of carbonic anhydrase and enhanced isozyme specificity is obtained.
Acknowledgement
ABM was supported by training grant T32AI0007110 from the NIH.
Abbreviations:
- CA
Carbonic Anhydrase
- ZBS
zinc-bound solvent
- ZBG
zinc-binding group
- SAC
saccharin
- SBC
saccharin-based compound
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Supporting Information
Spectroscopic data for compounds 1a–c, 3,4,5, and 8a–c.
Bibliography
- [1].Boone CD, Pinard M, McKenna R, Silverman D, Subcell Biochem, 75 (2014) 31–52. [DOI] [PubMed] [Google Scholar]
- [2].Lindskog S, in: Gas Enzymology, Springer, Dordrecht, 1985, pp. 121–133. [Google Scholar]
- [3].Supuran CT, Biochem J, 473 (2016) 2023–2032. [DOI] [PubMed] [Google Scholar]
- [4].Frost SC, Subcell Biochem, 75 (2014) 9–30. [DOI] [PubMed] [Google Scholar]
- [5].Henry RP, Annu Rev Physiol, 58 (1996) 523–538. [DOI] [PubMed] [Google Scholar]
- [6].Hewett-Emmett D, Tashian RE, Mol Phylogenet Evol, 5 (1996) 50–77. [DOI] [PubMed] [Google Scholar]
- [7].Eriksson AE, Jones TA, Liljas A, Proteins, 4 (1988) 274–282. [DOI] [PubMed] [Google Scholar]
- [8].Lindskog S, Pharmacol Ther, 74 (1997) 1–20. [DOI] [PubMed] [Google Scholar]
- [9].Aggarwal M, Kondeti B, McKenna R, Expert Opin Ther Pat, 23 (2013) 717–724. [DOI] [PubMed] [Google Scholar]
- [10].Alterio V, Di Fiore A, D’Ambrosio K, Supuran CT, De Simone G, Chem Rev, 112 (2012) 4421–4468. [DOI] [PubMed] [Google Scholar]
- [11].Guler OO, De Simone G, Supuran CT, Curr Med Chem, 17 (2010) 1516–1526. [DOI] [PubMed] [Google Scholar]
- [12].Mincione F, Scozzafava A, Supuran CT, Curr Top Med Chem, 7 (2007) 849–854. [DOI] [PubMed] [Google Scholar]
- [13].Supuran CT, Nature Reviews Drug Discovery, 7 (2008) 168–181. [DOI] [PubMed] [Google Scholar]
- [14].Chiche J, Ilc K, Laferriere J, Trottier E, Dayan F, Mazure NM, Brahimi-Horn MC, Pouyssegur J, Cancer Res, 69 (2009) 358–368. [DOI] [PubMed] [Google Scholar]
- [15].Lou Y, McDonald PC, Oloumi A, Chia S, Ostlund C, Ahmadi A, Kyle A, Auf dem Keller U, Leung S, Huntsman D, Clarke B, Sutherland BW, Waterhouse D, Bally M, Roskelley C, Overall CM, Minchinton A, Pacchiano F, Carta F, Scozzafava A, Touisni N, Winum JY, Supuran CT, Dedhar S, Cancer Res, 71 (2011) 3364–3376. [DOI] [PubMed] [Google Scholar]
- [16].Hockel M, Vaupel P, J Natl Cancer Inst, 93 (2001) 266–276. [DOI] [PubMed] [Google Scholar]
- [17].Moulder JE, Rockwell S, Cancer Metastasis Rev, 5 (1987) 313–341. [DOI] [PubMed] [Google Scholar]
- [18].Warburg O, J. Cancer Res, 9 (1925) 148–163. [Google Scholar]
- [19].McDonald PC, Winum JY, Supuran CT, Dedhar S, Oncotarget, 3 (2012) 84–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Zatovicova M, Jelenska L, Hulikova A, Csaderova L, Ditte Z, Ditte P, Goliasova T, Pastorek J, Pastorekova S, Curr Pharm Des, 16 (2010) 3255–3263. [DOI] [PubMed] [Google Scholar]
- [21].Neri D, Supuran CT, Nature Reviews Drug Discovery, 10 (2011) 767–777. [DOI] [PubMed] [Google Scholar]
- [22].Choueiri TK, Cheng S, Qu AQ, Pastorek J, Atkins MB, Signoretti S, Urol Oncol, 31 (2013) 1788–1793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Tafreshi NK, Lloyd MC, Bui MM, Gillies RJ, Morse DL, Subcell Biochem, 75 (2014) 221–254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Supuran CT, World J Clin Oncol, 3 (2012) 98–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Moeker J, Mahon BP, Bornaghi LF, Vullo D, Supuran CT, McKenna R, Poulsen SA, J Med Chem, 57 (2014) 8635–8645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Pinard MA, Mahon B, McKenna R, Biomed Res Int, 2015 (2015) 453543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Aggarwal M, Kondeti B, McKenna R, Bioorg Med Chem, 21 (2013) 1526–1533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Carta F, Temperini C, Innocenti A, Scozzafava A, Kaila K, Supuran CT, J Med Chem, 53 (2010) 5511–5522. [DOI] [PubMed] [Google Scholar]
- [29].Lopez M, Salmon AJ, Supuran CT, Poulsen SA, Curr Pharm Des, 16 (2010) 3277–3287. [DOI] [PubMed] [Google Scholar]
- [30].Maresca A, Temperini C, Vu H, Pham NB, Poulsen SA, Scozzafava A, Quinn RJ, Supuran CT, J Am Chem Soc, 131 (2009) 3057–3062. [DOI] [PubMed] [Google Scholar]
- [31].Woo LW, Ganeshapillai D, Thomas MP, Sutcliffe OB, Malini B, Mahon MF, Purohit A, Potter BV, ChemMedChem, 6 (2011) 2019–2034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Kohler K, Hillebrecht A, Schulze Wischeler J, Innocenti A, Heine A, Supuran CT, Klebe G, Angew Chem Int Ed Engl, 46 (2007) 7697–7699. [DOI] [PubMed] [Google Scholar]
- [33].Lomelino CL, Murray AB, Supuran CT, McKenna R, ACS Med Chem Lett, 9 (2018) 657–661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Murray AB, Lomelino CL, Supuran CT, McKenna R, J Med Chem, 61 (2018) 1176–1181. [DOI] [PubMed] [Google Scholar]
- [35].Pinard MA, Aggarwal M, Mahon BP, Tu C, McKenna R, Acta Crystallogr F Struct Biol Commun, 71 (2015) 1352–1358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Abdel-Aziz AAM, El-Azab AS, Abu El-Enin MA, Almehizia AA, Supuran CT, Nocentini A, Bioorg Chem, 80 (2018) 706–713. [DOI] [PubMed] [Google Scholar]
- [37].Hou Z, Lin B, Bao Y, Yan HN, Zhang M, Chang XW, Zhang XX, Wang ZJ, Wei GF, Cheng MS, Liu Y, Guo C, European Journal of Medicinal Chemistry, 132 (2017) 1–10. [DOI] [PubMed] [Google Scholar]
- [38].Mahon BP, Hendon AM, Driscoll JM, Rankin GM, Poulsen SA, Supuran CT, McKenna R, Bioorg Med Chem, 23 (2015) 849–854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Moeker J, Peat TS, Bornaghi LF, Vullo D, Supuran CT, Poulsen SA, J Med Chem, 57 (2014) 3522–3531. [DOI] [PubMed] [Google Scholar]
- [40].Liang LY, Astruc D, Coordin Chem Rev, 255 (2011) 2933–2945. [Google Scholar]
- [41].Tornoe CW, Christensen C, Meldal M, Journal of Organic Chemistry, 67 (2002) 3057–3064. [DOI] [PubMed] [Google Scholar]
- [42].Rostovtsev VV, Green LG, Fokin VV, Sharpless KB, Angew Chem Int Edit, 41 (2002) 2596–2599. [DOI] [PubMed] [Google Scholar]
- [43].Meldal M, Tornoe CW, Chemical Reviews, 108 (2008) 2952–3015. [DOI] [PubMed] [Google Scholar]
- [44].Tiwari VK, Mishra BB, Mishra KB, Mishra N, Singh AS, Chen X, Chemical Reviews, 116 (2016) 3086–3240. [DOI] [PubMed] [Google Scholar]
- [45].Hamor GH, Janfaza M, J Pharm Sci, 52 (1963) 102–103. [DOI] [PubMed] [Google Scholar]
- [46].Hoheisel TN, Frauenrath H, Org Lett, 10 (2008) 4525–4528. [DOI] [PubMed] [Google Scholar]
- [47].Zhao J, Liu Y, Park HJ, Boggs JM, Basu A, Bioconjug Chem, 23 (2012) 1166–1173. [DOI] [PubMed] [Google Scholar]
- [48].Hale KJ, Xiong Z, Wang L, Manaviazar S, Mackle R, Org Lett, 17 (2015) 198–201. [DOI] [PubMed] [Google Scholar]
- [49].Li X, Guo J, Asong J, Wolfert MA, Boons GJ, J Am Chem Soc, 133 (2011) 11147–11153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Uda NR, Seibert V, Stenner-Liewen F, Muller P, Herzig P, Gondi G, Zeidler R, van Dijk M, Zippelius A, Renner C, J Enzyme Inhib Med Chem, 30 (2015) 955–960. [DOI] [PubMed] [Google Scholar]
- [51].Baran EJ, Yilmaz VT, Coordin Chem Rev, 250 (2006) 1980–1999. [Google Scholar]
- [52].Lee YT, Cui CJ, Chow EW, Pue N, Lonhienne T, Wang JG, Fraser JA, Guddat LW, J Med Chem, 56 (2013) 210–219. [DOI] [PubMed] [Google Scholar]
- [53].Chowdhury MA, Abdellatif KR, Dong Y, Das D, Yu G, Velazquez CA, Suresh MR, Knaus EE, Bioorg Med Chem Lett, 19 (2009) 6855–6861. [DOI] [PubMed] [Google Scholar]
- [54].Kim SH, Tran MT, Ruebsam F, Xiang AX, Ayida B, McGuire H, Ellis D, Blazel J, Tran CV, Murphy DE, Webber SE, Zhou Y, Shah AM, Tsan M, Showalter RE, Patel R, Gobbi A, LeBrun LA, Bartkowski DM, Nolan TG, Norris DA, Sergeeva MV, Kirkovsky L, Zhao Q, Han Q, Kissinger CR, Bioorg Med Chem Lett, 18 (2008) 4181–4185. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.