Summary
Background and Aims
Emerging evidence shows that fibroblast growth factor 22 (FGF22) plays a critical role in the etiology of depression. However, the molecular mechanisms of FGF22 are not fully comprehended. Here, the effect of FGF22 in depression and its relationship with interleukin‐1β (IL‐1β) were investigated in clinical, animal, and cell experiments.
Methods
Serum from depressive patients was collected, and the levels of FGF22 and IL‐1β were analyzed by ELISA. The chronic unpredictable mild stress (CUMS) model was established, and primary hippocampal neuronal cells were cultured to examine changes in FGF22 and IL‐1β levels in rat hippocampus.
Results
The results revealed a negative correlation between serum FGF22 levels and serum IL‐1β levels. The expression of IL‐1β in the CUMS rat hippocampus decreased, and the apoptosis of hippocampal cells improved after the injection of lentiviral vector‐mediated FGF22 (LV‐FGF22). Further tests in primary hippocampal neuronal cells also showed a reduction in IL‐1β and the cell apoptosis rate after treatment with FGF22.
Conclusion
In conclusion, the results revealed that FGF22 plays a role in alleviating depression, which may be mediated by reduced expression of IL‐1β.
Keywords: depression, fibroblast growth factor 22, hippocampus, interleukin‐1β
1. INTRODUCTION
Depression is one of the most debilitating mental illness with enormous social and personal burdens.1 Several studies have been conducted, and many hypotheses produced around the pathogenesis of depression; however, the exact etiology and pathophysiology remain unclear.
The neurotrophic hypothesis has gained high attention of researchers,2 among other hypotheses of depression including the inflammatory hypothesis, HPA axis dysfunction, and others.3, 4 The neurotrophic hypothesis posits that the neurobiological basis for mood disorders may be due to the dysregulation of growth factors such as brain‐derived neurotrophic factors and fibroblast growth factors (FGFs), and the regulation of multiple neurotrophic factors may participate in the mechanisms underlying antidepressant effects.5 To date, representative neurotrophic factors of the FGF family, such as FGF2 and FGF9, which are well known to play significant roles in the formation and maintenance of synapse,6, 7 have been reported in the previous literature.8, 9 In addition, Terauchi showed that FGF22KO mice failed to form excitatory synapses in the hippocampus during development.6 Williams further found that FGF22KO mice exhibited an increased time of immobility, increased floating time, and reduced preference for sucrose,10 which suggested that the pathogenesis of depression involves a decrease in FGF22. These studies provide converging, complementary proof for the role of FGF22 in the rodent brain, and FGF22 as a new depression‐related neurotrophic factor, the mechanism of which is gradually being revealed in depressive disease.
Interleukin‐1β (IL‐1β) is not only a main driver of both peripheral and central immune responses but also the core factor of the inflammation hypothesis of depression.11 Clinical studies conducted by Rethorst found that serum IL‐1β levels in depressive patients were higher than in healthy controls, and IL‐1β levels decreased after antidepressant treatment.12, 13 Specifically, in animal experiments, after chronic unpredictable mild stress (CUMS), the gene expression of IL‐1β gene was significantly higher in rats’ hippocampus.14 Meanwhile, Park and his colleagues found that rats displayed depressive‐like behavior after IL‐1β intracerebroventricular injection,15 and Konsman found that IL‐1 receptor‐antagonist (IL‐1ra) leads to an antidepressant effect in rodent animals.16 Moreover, in cell experiments, Choi and Friedman found that the neurons of the hippocampus were vulnerable and prone to apoptosis after interfering with IL‐1β.17
The evidence above showed us that both FGF22 and IL‐1β are significantly involved in the pathogenesis of depression. Given our early observations that the levels of FGF22 and IL‐1β were altered in the serum of depressive patients, we further found the levels of FGF22 in the serum were negatively correlated with IL‐1β. Thus, we hypothesized that FGF22 can play a role in alleviating depression and the regulation of emotions and mood by decreasing the expression of IL‐1β. In addition, because it is difficult to determine the relationship between FGF22 and IL‐1β by serum index dysregulation in patients only, we used a series of animal experiments to further detect the role of FGF22 and corresponding changes of IL‐1β in depression. To that end, we also characterized the effects of FGF22 in primary hippocampal neuronal cells. Altogether, the combination of these various strands of evidence provides strong support that FGF22 take part in the nosogenesis of depression by reducing the expression of IL‐1β, which may be a possible target for novel treatment of depression.
2. MATERIALS AND METHODS
2.1. Participants
Ninety depressive patients were collected from the Affiliated Hospital of Jiangsu university. All diagnoses were made in accordance with the criteria of the Chinese classification of mental disorders‐third Edition (CCMD‐3). All patients collected should meet the following demands: (i) age 18‐65 years and han ethnicity, (ii) first episode and without drug treatment, (ii) HDRS‐24 ≥ 8. Patients with organic brain diseases, thyroid disease tumor, history of familial psychiatric disorders, or any other disease were excluded from this study. Patients in period of pregnancy or lactation were also excluded. Only venlafaxine was used as the therapeutic measure for the 90 patients during 8 weeks of treatment. Ninety healthy volunteers were recruited as controls. The Institutional Review Board of Affiliated Hospital of Jiangsu university ratified the research program, and the informed consent was signed by each of the participants.
2.2. Blood sample collection and serum FGF22 and IL‐1β measurement
After overnight fasting, venous blood (5 mL) from all participants was collected from 7 to 9 am in anticoagulant‐free tubes at baseline (w0). The blood samples were centrifuged at 1500 g for 20 minutes at 4°C to separate the serum after being kept for one hour at room temperature. The serum samples separated were aliquoted and kept at −80°C. The serum FGF22 and IL‐1β levels were measured using both human FGF22 (AVIVA SYSTEMS BIOLOGY, CAT OKEH02513) and IL‐1β (CUSABIO, CAT CBS‐E08053 h) ELISA kits according to manufacturer's instructions. The same blood collection and serum FGF22 and IL‐1β levels were determined again after treating with venlafaxine for 8 weeks (w8) for all patients. The concentration of FGF22 and IL‐1β was expressed as pg/ml.
2.3. Animals
All Wistar rats (sex: male, weight: 200 g‐250 g) were provided by Shanghai Slac Animal center, China. All rats were housed independently in standard plexiglas cages and under a normal 12‐h light/dark schedule. Before the action of the experiments, each of the rats was allowed 7 days to adapt to the living environment. Relative ambient temperature and humidity were maintained respectively at 22 ± 2°C and at 55 ± 5%. Each of the rats was given access to standard water and rat food during the process of the study, and the Institute for Experimental Animals of Jiangsu University approved all procedures of this study.
2.4. Chronic unpredictable mild stress (CUMS) program
The stress program was slightly adjusted.18 Each of the rats in CUMS groups was exposed to different stressors, including overnight illumination, 5 min cold swimming (at 4°C), 24 hours water deprivation, physical restraint for 2 hours, 24 hours food deprivation, 1 minute tail pinch, and exposure to rat odor for 1 hour, and one of these stressors was actualized every day in random order for 4 weeks. Identical conditions without stress in a separate room were used for unstressed rats. Before they were sacrificed, each of the rats was examined the behavioral test.
2.5. Lentivirus vectors (LV) and experimental animal grouping
The lentivirus‐derived plasmid (p3XFLAG‐Myc‐CMV‐23), which carried the transgenes for FGF22 (primers: 5′‐ CCCATGCGCCGCCGCCTC ‐3′, and 5′‐ TCAAGATGAG ACCAGTACTGGTAGGAACATG ‐3′), driven by the cytomegalovirus promoter, was used to overexpress the FGF22. In these experiments, to investigate the effects of hippocampal FGF22 overexpression on CUMS rats, rats were divided into three groups including CUMS group, CUMS group with Mock1 injection (CUMS+Mock1), and CUMS group with Lv‐FGF22 injection (CUMS+FGF22) (n = 6 for each group). The empty vector was used as Mock1. Moreover, to further validate whether the blocking of hippocampal IL‐1β receptor has equivalent effect with FGF22 overexpression, rats were divided into three groups including CUMS group, CUMS group with Mock2 injection (CUMS+Mock2), and CUMS group with IL‐1ra injection (CUMS+IL‐1ra) (n = 6 for each group). The normal saline was used as Mock2.
2.6. Stereotaxic injection of LV‐FGF22, Mock1, IL‐1ra, and Mock2
Viral injections (Lv‐FGF22, Mock1) were performed according to the previous study.19 A ketamine/xylazine mixture (0.1 g/kg and 0.01 g/kg, respectively, i.p.) was used to anesthetize for stereotaxic surgery. Rats were injected with viral solution bilaterally using a precision Hamilton microsyringe (26G) according to the coordinates as followed: hippocampus [1st injection: 6 mm ventral to the skull surface, 5 mm lateral the medial suture, 4.8 mm posterior to Bregma 2nd injection: 3.5 mm ventral to the skull surface, 2.5 mm lateral the medial suture, 4.8 mm posterior to Bregma.]. Each of the rats was exposed to CUMS after 7 days recovery.
ALZET osmotic pumps were used to keep the persistence of intrahippocampal injection of human recombinant IL‐1ra (PROSPECT, CAT CYT‐152). IL‐1ra(140 μg) diluted in sterile saline in osmotic pumps were used to block the IL‐1R in hippocampus constantly, and animals received 5 μg per day during 4 weeks.20 Meanwhile, the physiological activity and stability of IL‐1ra were proved to be preserved at 37°C as long as 4 weeks when used.21, 22
2.7. Body weights
As the previous study described,23 body weights were examined on the same time of each week prior to any procedure. To acquire the normalized body weights, the body weights of rats measured on each week were normalized to the first day to make comparison.
2.8. Open field exploration (OFE)
After 4 weeks of CUMS, the movements of the rats in each group were recorded in the object displacement task, which was similar to an OFE task as the previous study23 during the habituation phase. The blue lines mean the motion trail of the animal. The big black circle means the borderline of the arena. The gray domain (15 cm), which is one‐third the radius of the arena, means the center area of the arena. The path traveled and the time spent in the center domain were recorded for further analysis.
2.9. Forced swim test (FST)
Twenty‐four hours after the OFE, the FST was carried out as previously described.24 Rats were then placed into the glass beakers which were filled with tap water (22‐26°C) and had no way to touch the bottom of the beaker to escape or rest. The duration time of swimming and immobility was recorded (the observation period lasted 360 seconds) for further analysis.
2.10. Immunohistochemistry
The expression of IL‐1β proteins in the hippocampal tissue was detected by immunostaining. Firstly, the paraffin wax‐embedded hippocampus was dewaxed and treated with 3% H2O2 for 5 minutes to restrain the peroxidase. Afterward, the sections were washed with distilled water, and washed by phosphate‐buffered saline (5 minutes), and incubated with IL‐1β antibody (CUSABIO, CAT CBS‐PA003023) diluent (15 minutes). Then the sections were washed three times (5 minutes per time) with phosphate‐buffered saline and incubated with secondary antibody diluent (30 minutes). Before being incubated with streptavidin‐HRP complex, the sections were washed three times (3 minutes per time) with phosphate‐buffered saline. After being washed another three times (5 minutes per time) with phosphate‐buffered saline, 3,3′‐diaminobenzidine (DAB) solution was added to the reaction for 10 minutes. Finally, gelatin was used to envelop the sections which were observed under the microscope. To count the cells, each section was observed and photographed at a magnification of 200, and the number of immune‐positive cells was calculated per square millimeter in the target area. The Image‐Pro Plus system (Media Cybernetics, Silver Spring, MD, USA) was used to analyze the cell.
2.11. Primary hippocampal neuronal cultures
Firstly, hippocampus was separated from rat embryo brain carefully as originally reported.25 After being dissociated with trypsin (Gibco, Grand Island, NY, USA) digestion, the neuronal cells were suspended again in a neurobasal medium (Gibco) (B27 19 final, Gibco) containing 2 mmol/L GlutaMAX. Then cells were diluted to 100 cells/mL and almost 300 cells (3 mL cell suspension) were put into polyD‐lysine‐coated plate (35 mm). The plate was cultured in 5% CO2 at 37°C for 120 minutes. Afterward, 3 mL of neurobasal/B27 medium was used to replace the old plating medium. Before treatment, the primary cells were permitted to culture for 14 days. The expression levels of IL‐1β and apoptosis protein in the cultured neuronal cells were able to be analyzed after being treated with FGF22 for 24 hours.
2.12. Western blot
Proteins (40‐50 mg) were separated from cells or tissues by SDS‐PAGE as previously described.26 The proteins were transferred to polyvinylidene difluoride membranes, and then the membranes were blocked. The first antibodies against FGF22 (AVIVA SYSTEM BIOLOGY, CAT OAAF02973), IL‐1β (CUSABIO, CAT CBS‐PA003023), Bcl‐2 (AVIVA SYSTEM BIOLOGY, OAAF00934), Bad (AVIVA SYSTEM BIOLOGY, CAT OAAF01244), and β‐actin (AVIVA SYSTEM BIOLOGY, CAT OAAB19522) were used to probe. Horseradish peroxidase‐conjugated secondary antibodies were used to incubate the blot after washing, and the result of FGF22, IL‐1β, Bcl‐2, and Bad were normalized to β‐actin.
2.13. Flow cytometry
Primary hippocampal neuronal cells were seeded at 4.0 × 105 cells/well in 6‐well plates and cultured in DMEM containing FGF22 100 μg/L or 200 ug/L for 24 hours. Cells cultured in DMEM without FGF22 for 24 hours served as controls. After treatment, the cells were washed twice with ice‐cold PBS. The neuronal cells were then resuspended in binding buffer (100 μL) which contained 5 μL of PI and 5 μL of Annexin V‐FITC stock (Annexin V‐FITC kit, Becton‐Dickinson, San Jose, CA, USA). Afterward, 400 μL binding buffer was added and mixed gently after incubation at room temperature (15 minutes) in the dark. Flow cytometry was used to analyze the sample, and the apoptotic cells (annexin V+/PI‐ cells) were analyzed with CellQuest software (Becton‐Dickinson).
2.14. Statistical analysis
All data were analyzed by GraphPad Prism 5 (GraphPad Software, Inc., La Jolla, CA, USA) or SPSS (IBM Corporation, Armonk, New York, NY, USA) and presented as mean±SD. Clinical data were analyzed by t tests and Chi‐square test, and the relationship among serum FGF22, IL‐1β levels, and clinical HDRS scores was assessed using Spearman's correlation coefficient. Animal and cell data were analyzed using one‐way or two‐way analysis of variance (ANOVA) followed by Bonferroni posttests multiple comparison test when F values were significant. The difference was considered to be significant at P < 0.05.
3. RESULTS
3.1. Changes of serum FGF22 and IL‐1β levels in depressive patients
The participants’ demographic and clinical data are listed in Table S1 and the difference between DD and HC did not show statistically significant in the sex, age, height, weight, and years of schooling. As shown in Figure 1A‐D, the patients presented significantly lower serum FGF22 levels (180.44 ± 17.02 vs 200.74 ± 16.63, t(179) = 8.090, P < 0.01) but higher serum IL‐1β levels (11.23 ± 1.89 vs 5.22 ± 1.60, t(179) = 23.02, P < 0.01) compared to HC at baseline, and the patients’ serum FGF22 levels increased (w0 = 180.44 ± 17.02; w8 = 195.74 ± 19.57, t(179) = 13.18, P < 0.01) while IL‐1β levels decreased (w0 = 11.23 ± 1.89, w8 = 8.16 ± 1.50, t(179) = 15.82, P < 0.01) after 8 weeks of treatment. The correlation analysis showed that serum FGF22 levels were negative correlated with HDRS scores (r = −0.691, P < 0.01) (Figure 1E) and serum IL‐1β levels (r = −0.685, P < 0.01) (Figure 1F). Meanwhile, serum IL‐1β levels were positively correlated with HDRS scores (r = 0.749, P < 0.01) (Figure 1G).
Figure 1.
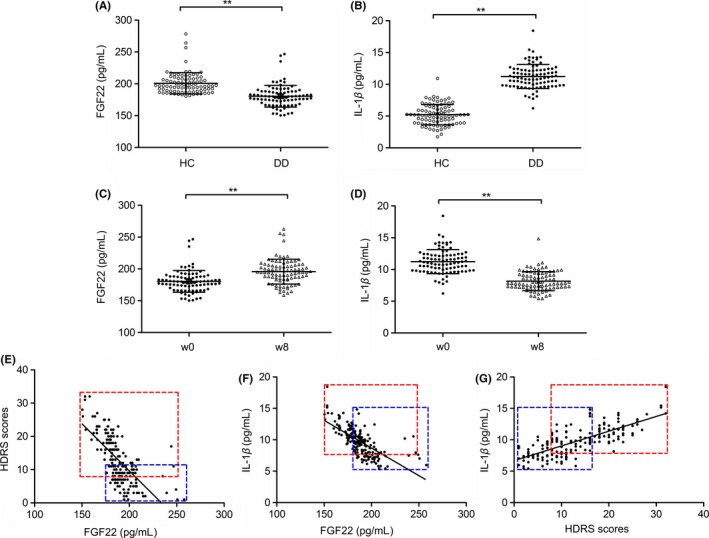
Alteration of serum FGF22 and IL‐1β levels. Serum FGF22 levels (A) and IL‐1β levels (B) in DD patients and healthy controls at baseline (Unpaired t test). Changes in serum FGF22 levels (C) and IL‐1β levels (D) in DD patients after an 8‐week antidepressant treatment (Paired t test). E, Negative correlation between serum FGF22 levels and HDRS scores (Spearman's correlation analysis). F, Negative correlation between serum FGF22 levels and serum IL‐1β levels (Spearman's correlation analysis). G, Positive correlation between serum IL‐1β levels and HDRS scores (Spearman's correlation analysis). The red frame represents w0 and the blue frame represents w8, *P < 0.05, **P < 0.01
3.2. Reduction in FGF22 and upregulation of IL‐1β in rat hippocampus induced by CUMS
We examined our CUMS model by assessing its impact on body weight, OFE, FST, and the size of hippocampus (Fig. S1), which provided us with abundant evidence of building the model successfully. During the 4 weeks of establishing our CUMS models, we analyzed the expression of FGF22 and IL‐1β in the hippocampus of CUMS rats. We found that CUMS induced decreased FGF22 expression in the hippocampus with exposure to stress in a time‐dependent manner, while IL‐1β expression in the hippocampus increased (Figure 2A‐C).
Figure 2.
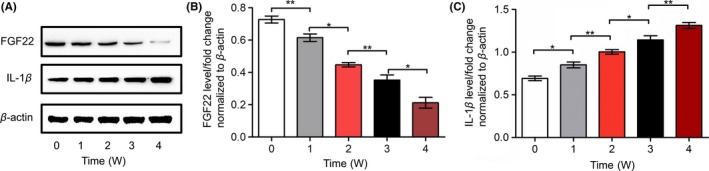
Alteration of FGF22 and IL‐1β expression in rat hippocampi induced by CUMS over the time points shown. A, Protein levels of FGF22 and IL‐1β shown with representative Western blot images. B, Quantitative analysis of FGF22 protein levels normalized to β‐actin. C, Quantitative analysis of IL‐1β protein levels normalized to β‐actin. n = 6, *P < 0.05, **P < 0.01 (one‐way ANOVA, Bonferroni posttests)
3.3. Hippocampal FGF22 overexpression alleviated CUMS‐induced depression‐like behavior and apoptosis in the hippocampus
In these experiments, rats were tested with body weights, OFE, FST, and apoptotic protein in the hippocampus. Bonferroni posttests evaluations revealed that there was no difference between CUMS group and CUMS+Mock1 group in these tests (Figure 3A‐J). The changes in body weight [main effects of groups (F (2,75) = 308.50, P < 0.01); time (F (4,75) = 12.15, P < 0.01); and interaction (F (8,75) = 47.88, P < 0.01)] are shown in Figure 3A, indicating that the CUMS+FGF22 group had a greater percentage weight gained across weeks (P < 0.01) compared to CUMS and CUMS+Mock1 groups. Meanwhile, in the OFE, the CUMS+FGF22 group showed more time in the center area (P < 0.05) and a higher percentage of the path traveled in the center (P < 0.01) compared to the another two groups (Figure 3D‐E). However, Figure 3C showed no difference in the total path traveled. Tests in FST showed that CUMS+FGF22 group spent more time on swimming (P < 0.01) and less time on immobility (P < 0.05) compared to another two groups (Figure 3F‐G). Behavioral testing showed us that overexpressing FGF22 can alleviate the depression‐like behavior induced by CUMS. Moreover, we determined both antiapoptotic protein Bcl‐2 and proapoptotic protein Bad levels to investigate the effect of FGF22 on the hippocampal apoptosis. Our findings showed that the CUMS+FGF22 group exhibited higher Bcl‐2 levels (P < 0.05) and lower Bad levels (P < 0.05) than the CUMS and CUMS+Mock1 groups (Figure 3H‐J), suggesting that FGF22 can alleviate apoptosis in the hippocampus.
Figure 3.
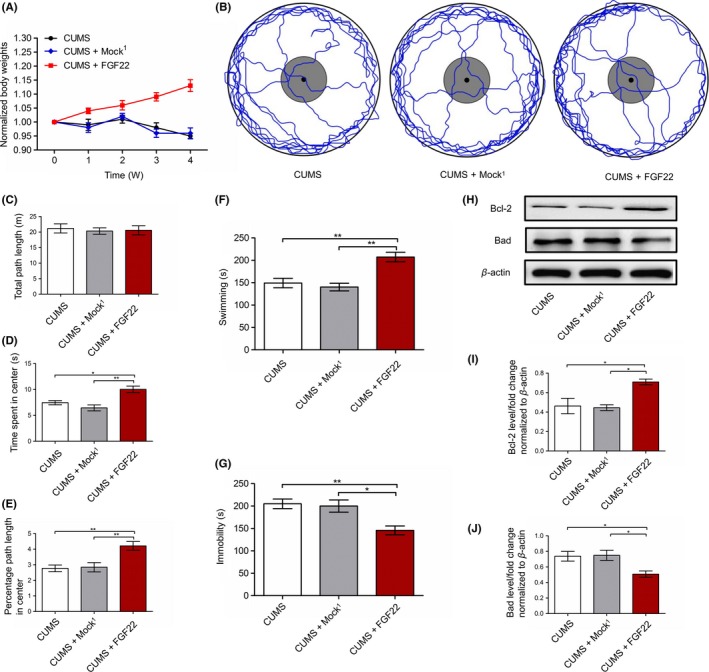
Effect of lentiviral‐mediated FGF22 overexpression on CUMS‐induced depressive manifestation and hippocampal apoptosis. A, Body weights normalized to the body weight on day 1 (the day stress commenced) for all the animals (two‐way ANOVA, Bonferroni posttests). B, Representative tracks depicting open field exploration. C, Total path length traveled. D, Time spent in the center area. E, Percentage path length traveled in the center area. F, Swimming in FST. G, Immobility in FST. H, Protein levels of Bcl‐2 and Bad shown with representative Western blot images. I, Quantitative analysis of Bcl‐2 protein levels normalized to β‐actin. J, Quantitative analysis of Bad protein levels normalized to β‐actin. n = 6, *P < 0.05, **P < 0.01 (one‐way ANOVA, Bonferroni posttests)
3.4. FGF22 downregulates IL‐1β protein expression in hippocampus
As our preliminary results showed the reduction in FGF22 with IL‐1β upregulation and a clinical negative correlation between the two, we speculated that IL‐1β levels might respond to FGF22 changes, and LV‐FGF22 was used to enhance the expression of FGF22 in rat hippocampus. Higher FGF22 levels (P < 0.01) and lower IL‐1β levels (P < 0.01) were shown in the CUMS+FGF22 group compared to the another two groups (Figure 4A‐C). Meanwhile, immunohistochemistry of hippocampi also showed a decrease of IL‐1β in CA1, CA2, and DG area after LV‐FGF22 injection (P < 0.01) (Figure 4D‐E).
Figure 4.
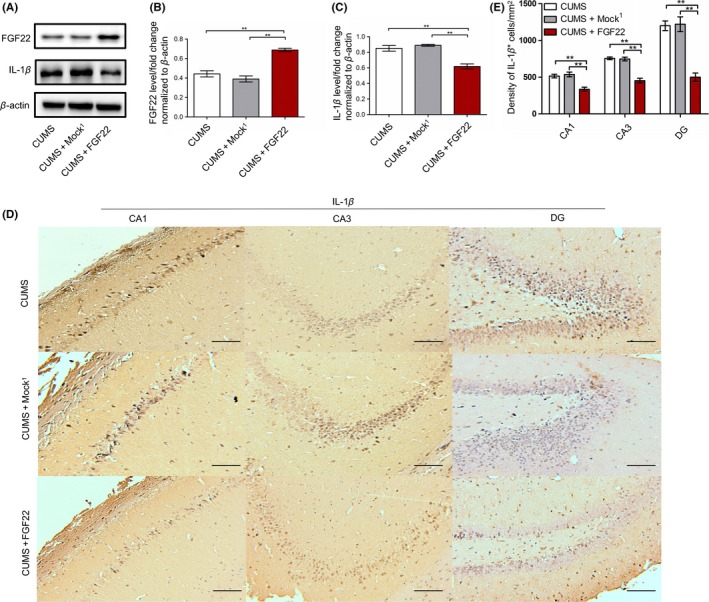
The changes in FGF22 and IL‐1β levels in rat hippocampus after LV‐FGF22 treatment. A, Protein levels of FGF22 and IL‐1β shown with representative Western blot images. B‐C, Quantitative analysis of FGF22 and IL‐1β protein levels normalized to β‐actin. D, Representative photomicrographs were captured, showing immunostaining for IL‐1β in the CA1, CA3, and DG in three groups responding to LV‐FGF22 treatment. Bar=100 μm. E, Quantitative analysis of IL‐1β‐positive cells in the CA1, CA3, and DG. n = 6, *P < 0.05, **P < 0.01 (one‐way ANOVA, Bonferroni posttests)
3.5. The effects of continuous intrahippocampal delivery of IL‐1ra on behavior and apoptosis in the hippocampus
To examine whether the blocking of hippocampal IL‐1β receptor has the same effects as the overexpression of FGF22 on depression, the CUMS rats were injected with IL‐1ra in the hippocampus. As shown in Figure 5A, the CUMS+IL‐1ra group showed an increase in percentage weight gained compared to the CUMS and CUMS+Mock2 groups (P < 0.01). In the OFE (Figure 5B‐E), the CUMS+IL‐1ra group spent more time in the center of the arena (P < 0.01) and traveled a higher percentage of the path in the center (P < 0.05) compared to the another two groups. There was no difference in the total path traveled among three groups. Afterward, tests in FST showed that the CUMS and CUMS+Mock2 groups spent less time on swimming (P < 0.01) and more time on immobility (P < 0.05) compared to CUMS+IL‐1ra group (Figure 5F‐G). We then determined the levels of Bcl‐2 and Bad protein in hippocampus, which showed higher Bcl‐2 levels (P < 0.05) and lower Bad levels (P < 0.01) in the CUMS+IL‐1ra group compared to the another two groups (Figure 5H‐J).
Figure 5.
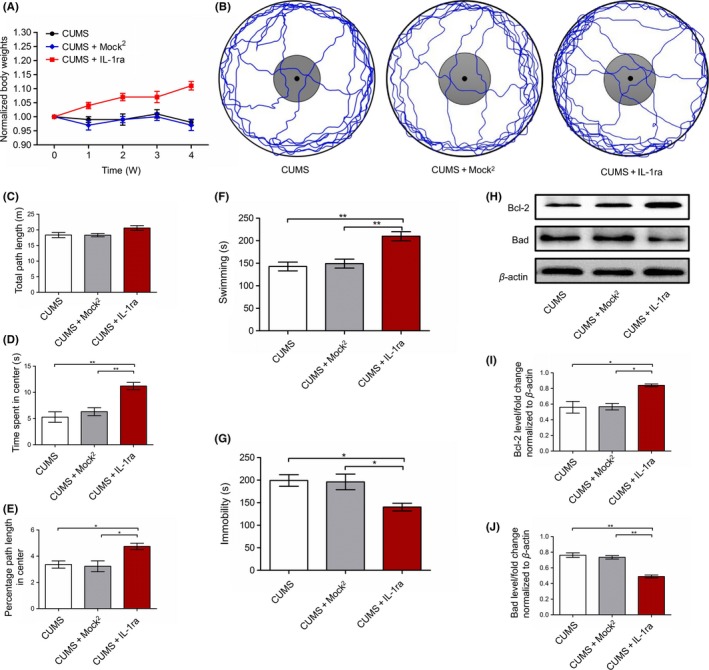
Effect of continuous intrahippocampal delivery of IL‐1ra on CUMS‐induced depressive manifestation and hippocampal apoptosis. A, Body weights normalized to the body weight on day 1 (the day stress commenced) for all the animals (two‐way ANOVA, Bonferroni posttests). B, Representative tracks depicting open field exploration. C, Total path length traveled. D, Time spent in the center area. E, Percentage of the path length traveled in the center area. F, Swimming in FST. G, Immobility in FST. H, Protein levels of Bcl‐2 and Bad shown with representative Western blot images. I, Quantitative analysis of Bcl‐2 protein levels normalized to β‐actin. J, Quantitative analysis of Bad protein levels normalized to β‐actin. n = 6, *P < 0.05, **P < 0.01 (one‐way ANOVA, Bonferroni posttests)
3.6. FGF22 downregulates IL‐1β protein expression in primary hippocampal neuronal cells
To further validate whether FGF22 could reduce expression of IL‐1β, primary hippocampal neuronal cells were also used. FGF22 (100 μg/L) or FGF22 (200 μg/L) was added to the culture medium, and the levels of IL‐1β, Bcl‐2, and Bad were characterized. Our results showed that after treated with FGF22, the expression of IL‐1β and Bad was downregulated, and Bcl‐2 was upregulated. The changes of IL‐1β, Bcl‐2, and Bad protein induced by FGF22 in a dose‐dependent manner (Figure 6A‐D). Moreover, flow cytometry also showed that cell treated with FGF22 (200 μg/L) exhibited less apoptosis (P < 0.01) compared to those treated with FGF22 (100 μg/L; Figure 6E‐H), which also suggested that the apoptosis rate of primary hippocampal neuronal cells was significantly suppressed in the presence of FGF22.
Figure 6.
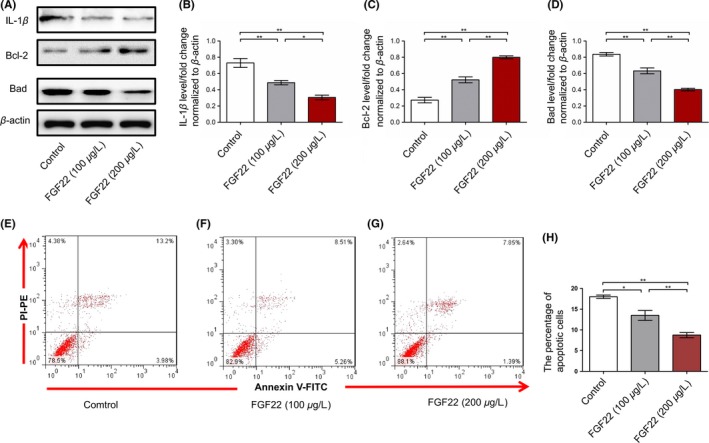
Effect of FGF22 on primary hippocampal neuronal cells. A, IL‐1β, Bcl‐2, and Bad protein alteration in the cultured hippocampal neurons. B‐D, Quantitative analysis of IL‐1β, Bcl‐2, and Bad protein levels normalized to β‐actin. E‐G, Determination of apoptosis with the annexin V‐propidium iodide PI dual staining assay in primary hippocampal neurons. H, The percentage distribution of apoptotic cells. n = 6, *P < 0.05, **P < 0.01 (one‐way ANOVA, Bonferroni posttests)
4. DISCUSSION
FGF22 has been closely associated with the nosogenesis of many neuropsychiatric diseases.27 However, the role of FGF22 in depression is not yet clear. Here, we determined the function of FGF22 using clinical, animal, and cell studies and demonstrated, for the first time, that the function of FGF22 in depression may be executed by reducing IL‐1β expression.
So far, research on the relationship between depression and FGF22 is still limited. The existing research on FGF22KO mice showed the undevelopment of excitatory synapses in the hippocampus, which could make explanation for the depressive behaviors.10 Previous animal studies on the FGF gene knockout mice showed that FGF2KO mice could exhibit depression‐like behaviors,28 and corresponding clinical studies conducted by Shen showed that the serum FGF2 levels were lower in depressive patients than in healthy controls.9 On this basis, we wondered about changes in FGF22 in depressive patients and determined the serum FGF22 levels in patients by ELISA due to the limitations to obtaining the hippocampus samples from human patients. Our results paralleled the changes of serum FGF2 levels and suggested that FGF22 might serve as a potential peripheral biomarker in depression. Moreover, the negative correlation between serum FGF22 levels and IL‐1β levels sparked our interest in the relationship between FGF22 and IL‐1β.
FGFs have complex relationships with interleukins, which have been extensively researched in many diseases. Schaefer put forward that FGF signaling may influence the function of IL‐1β and found that FGF had antagonistic effects on IL‐1β in chondrosarcoma cells.29 Similarly, Ruiz and his colleagues found that the expression of cytokines such as IL‐4 and IL‐12 was reduced after treatment with FGF in cultured human osteoblast‐like cells,30 suggesting that FGFs might inhibit the expression of interleukin in several diseases. Taking this evidence and our clinical results into consideration, we put forward the hypothesis that FGF22 may reduce the expression of IL‐1β in depression, which led us to do further animal and cell experiments to verify.
In the animal experiments, we first found the reduction in FGF22 and upregulation of IL‐1β in CUMS rats exhibited in a time‐dependent manner, which was consistent with our clinical serological results. Meanwhile, our research found that CUMS+FGF22 group exhibited an alleviation of depression‐like behaviors and apoptosis of hippocampus. We then detected the content of IL‐1β in CUMS+FGF22 group and validated that IL‐1β was regulated by the content of FGF22. Afterward, we also examined the depression‐like behaviors and apoptosis of hippocampus in CUMS+IL‐1ra group and demonstrated that blocking the expression of IL‐1β can phenocopy the antidepressant‐like effects of FGF22 overexpression. Moreover, in cell experiments, we found that IL‐1β was reduced after the addition of FGF22 in cultured primary hippocampal neuronal cells, and the apoptosis rate was also lessened. These results collectively showed that FGF22 plays an antidepressive role in the disorder and exerts its function by downregulating IL‐1β expression.
In summary, the current study lends further support for FGF22 as a potential IL‐1β modulator. It should be noted that factors like duration of illness and severity may have effects on the results of antidepressant treatment for patients. A further limitation was that our multiple comparison correction method of Bonferroni correction was a less conservative method compared to a false discovery rate. However, considering that Bonferroni correction is widely used and demonstrated to be better when the comparison conducted only a small number of times, we believed it might be a sufficient statistical tool for correcting multiple comparisons in this study. Additionally, we have only conducted a preliminary study of the mechanism of FGF22 in depression and demonstrated it can modulate depression although IL‐1β, and FGF22 may also have other effects in depression which need further exploration. Notwithstanding its limitations, this study does suggest the importance and the possible mechanism of FGF22 in depression, which provides a new direction to research the pathogenesis and treatment of depression.
5. CONCLUSION
This study showed that serum FGF22 levels in depressive patients were negatively correlated with serum IL‐1β levels. In animal and cell experiments, enhancing the levels of FGF22 in rat hippocampus was demonstrated to alleviate the CUMS‐induced depression. The increase in FGF22 was found to be associated with reduced IL‐1β expression and hippocampal apoptosis, suggesting a necessary investigation of FGF22 as a potential IL‐1β modulator.
CONFLICT OF INTEREST
The authors declare no conflict of interest.
Supporting information
ACKNOWLEDGMENTS
This program was supported by Jiangsu Provincial Key Research and Development Plan (BE2017698), project of Jiangsu Provincial Health and Family Planning Commission (Q201605), project of Jiangsu Provincial young medical talents (QNRC2016832/QNRC2016460).
Xu Y‐H, Yu M, Wei H, et al. Fibroblast growth factor 22 is a novel modulator of depression through interleukin‐1β. CNS Neurosci Ther. 2017;23:907–916. 10.1111/cns.12760
The first two authors contributed equally to this work.
Contributor Information
Xiao‐Lan Zhu, Email: 13775552517@163.com.
Yue‐Feng Li, Email: jiangdalyf@163.com.
REFERENCES
- 1. Caraceno B. Strategic directions and activity review of the Department of Mental Health and Substance Abuse of WHO. Zh Nevrol Psikhiatr Im S S Korsakova. 2006;106:74‐76. [PubMed] [Google Scholar]
- 2. Buttenschøn HN, Foldager L, Elfving B, Poulsen PH, Uher R, Mors O. Neurotrophic factors in depression in response to treatment. J Affect Disord. 2015;183:287‐294. [DOI] [PubMed] [Google Scholar]
- 3. Pariante CM. Risk factors for development of depression and psychosis. Glucocorticoid receptors and pituitary implications for treatment with antidepressant and glucocorticoids. Ann N Y Acad Sci. 2009;1179:144‐152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kohler CA, Freitas TH, Maes M, et al. Peripheral cytokine and chemokine alterations in depression: a meta‐analysis of 82 studies. Acta Psychiatr Scan. 2017;135:373‐387. [DOI] [PubMed] [Google Scholar]
- 5. Turner CA, Watson SJ, Akil H. The fibroblast growth factor family: neuromodulation of affective behavior. Neuron. 2012;76:160‐174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Terauchi A, Johnson‐Venkatesh EM, Toth AB, Javed D, Sutton MA, Umemori H. Distinct FGFs promote differentiation of excitatory and inhibitory synapses. Nature. 2010;465:783‐787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Umemori H. Weaving the neuronal net with target‐derived fibroblast growth factors. Dev Growth Differ. 2009;51:263‐270. [DOI] [PubMed] [Google Scholar]
- 8. Aurbach EL, Inui EG, Turner CA, et al. Fibroblast growth factor 9 is a novel modulator of negative affect. Proc Natl Acad Sci USA. 2015;112:11953‐11958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. He S, Zhang T, Hong B, et al. Decreased serum fibroblast growth factor ‐ 2 levels in pre‐ and post‐treatment patients with major depressive disorder. Neurosci Lett. 2014;579:168‐172. [DOI] [PubMed] [Google Scholar]
- 10. Williams AJ, Yee P, Smith MC, Murphy GG, Umemori H. Deletion of fibroblast growth factor 22 (FGF22) causes a depression‐like phenotype in adult mice. Behav Brain Res. 2016;307:11‐17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. van de Veerdonk FL, Netea MG. New Insights in the Immunobiology of IL‐1 Family Members. Front Immunol. 2013;4:167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hannestad J, DellaGioia N, Bloch M. The effect of antidepressant medication treatment on serum levels of inflammatory cytokines: a meta‐analysis. Neuropsychopharmacology. 2011;36:2452‐2459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Rethorst CD, Toups MS, Greer TL, et al. Pro‐inflammatory cytokines as predictors of antidepressant effects of exercise in major depressive disorder. Mol Psychiatry. 2013;18:1119‐1124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Wang N, Yu HY, Shen XF, et al. The rapid antidepressant effect of ketamine in rats is associated with down‐regulation of pro‐inflammatory cytokines in the hippocampus. Upsala J Med Sci. 2015;120:241‐248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Park HJ, Shim HS, An K, Starkweather A, Kim KS, Shim I. IL‐4 Inhibits IL‐1beta‐Induced Depressive‐Like Behavior and Central Neurotransmitter Alterations. Mediators Inflamm. 2015;2015:941413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Terauchi A, Johnson‐Venkatesh EM, Toth AB, Javed D, Sutton MA, Umemori H. Central nervous action of interleukin‐1 mediates activation of limbic structures and behavioural depression in response to peripheral administration of bacterial lipopolysaccharide. Eur J Neurosci. 2008;28:2499‐2510. [DOI] [PubMed] [Google Scholar]
- 17. Choi S, Friedman WJ. Interleukin‐1beta enhances neuronal vulnerability to proNGF‐mediated apoptosis by increasing surface expression of p75(NTR) and sortillin. Neuroscience. 2014;257:11‐19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Yi LT, Li J, Liu BB, Luo L, Liu Q, Geng D. BDNF‐ERK‐CREB signalling mediates the role of miR‐132 in the regulation of the effects of oleanolic acid in male mice. J Psychiatry Neurosci. 2014;39:348‐359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Bahi A, Chandrasekar V, Dreyer JL. Selective lentiviral‐mediated suppression of microRNA124a in the hippocampus evokes antidepressants‐like effects in rats. Psychoneuroendocrinology. 2014;46:78‐87. [DOI] [PubMed] [Google Scholar]
- 20. Vezzani A, Moneta D, Conti M, et al. Powerful anticonvulsant action of IL‐1 receptor antagonist on intracerebral injection and astrocytic overexpression in mice. Proc Natl Acad Sci USA. 2000;97:11534‐11539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Knedla A, Riepl B, Lefevre S, et al. The therapeutic use of osmotic minipumps in the severe combined immunodeficiency (SCID) mouse model for rheumatoid arthritis. Ann Rheum Dis. 2009;68:124‐129. [DOI] [PubMed] [Google Scholar]
- 22. Sandberg JO, Eizirik DL, Sandler S, Tracey DE, Andersson A. Treatment with an interleukin‐1 receptor antagonist protein prolongs mouse islet allograft survival. Diabetes. 1993;42:1845‐1851. [DOI] [PubMed] [Google Scholar]
- 23. Rahman MM, Callaghan CK, Kerskens CM, Chattarji S, O'Mara SM. Early hippocampal volume loss as a marker of eventual memory deficits caused by repeated stress. Sci Rep. 2016;6:29127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Cao W, Duan J, Wang X, et al. Early enriched environment induces an increased conversion of proBDNF to BDNF in the adult rat's hippocampus. Behav Brain Res. 2014;265:76‐83. [DOI] [PubMed] [Google Scholar]
- 25. Fath T, Ke YD, Gunning P, Gotz J, Ittner LM. Primary support cultures of hippocampal and substantia nigra neurons. Nat Protoc. 2009;4:78‐85. [DOI] [PubMed] [Google Scholar]
- 26. Hou G, Tang M, Yan L, Wang M. Changes of ultrastructure and downregulation of heat shock protein 70 and fibroblast growth factor 2 in gastric mucosa of rats with depressive‐like behaviour. Acta Neuropsychiatr. 2012;24:160‐165. [DOI] [PubMed] [Google Scholar]
- 27. Umemori H, Linhoff MW, Ornitz DM, Sanes JR. FGF22 and its close relatives are presynaptic organizing molecules in the mammalian brain. Cell. 2004;118:257‐270. [DOI] [PubMed] [Google Scholar]
- 28. Salmaso N, Stevens HE, McNeill J, et al. Fibroblast Growth Factor 2 Modulates Hypothalamic Pituitary Axis Activity and Anxiety Behavior Through Glucocorticoid Receptors. Biol Psychiat. 2016;80:479‐489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Schaefer JF, Millham ML, de Crombrugghe B, Buckbinder L. FGF signaling antagonizes cytokine‐mediated repression of Sox9 in SW1353 chondrosarcoma cells. Osteoarthritis Cartilage. 2003;11:233‐241. [DOI] [PubMed] [Google Scholar]
- 30. Ruiz C, Perez E, Garcia‐Martinez O, Diaz‐Rodriguez L, Arroyo‐Morales M, Reyes‐Botella C. Expression of cytokines IL‐4, IL‐12, IL‐15, IL‐18, and IFNgamma and modulation by different growth factors in cultured human osteoblast‐like cells. J Bone Miner Metab. 2007;25:286‐292. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


