Summary
Aims
Acute postoperative pain remains a significant healthcare issue. Historically, the assessment of postoperative pain in rodents has relied on evoked withdrawal or reflexive measures. Using a recently developed, anatomically relevant rat model of acute postoperative pain (J Pain, 16, 2015, 421), the present experiments sought to investigate the affective component of acute postoperative pain associated with inguinal hernia repair.
Methods
Male Lister hooded rats underwent surgery to model Lichtenstein inguinal hernia repair (without hernia induction), or a sham procedure. Postsurgical characterization involved a modified place escape/avoidance paradigm (mPEAP), as well as home cage and open field locomotor activity monitoring. In pharmacological validation studies, rats received either morphine or carprofen prior to mPEAP testing.
Results
Surgery was associated with a significantly increased proportion of the trial duration in the light compartment of the mPEAP arena, in avoidance of the noxious stimulus, compared with sham animals. When retested in the mPEAP at day 7 postsurgery, there was no difference between sham and surgery animals for time spent in either compartment, but surgery animals displayed a persistent increase in the percentage response to noxious stimulation. Morphine and carprofen treatment in surgery animals reduced escape/avoidance behavior at discrete time points over the trial. Surgery‐induced reductions in home cage and open field locomotor activity were also observed.
Conclusion
The present studies report for the first time the characterization of the affective component of acute postoperative pain using the mPEAP in a rodent model, which may facilitate development of improved understanding and treatment of postoperative pain.
Keywords: Affect, Carprofen, Hernia repair, Locomotor activity, Morphine, PEAP, Postsurgical pain
Introduction
Up to 41% of patients who undergo surgery experience moderate‐to‐severe acute postoperative pain despite the use of acute pain management protocols 1. Inguinal hernia repair is one of the most common surgical procedures 2 with approximately 40% of patients experiencing moderate‐to‐severe acute pain in the early hours and days following surgery that can negatively impact on their physical and mental well‐being 3, 4, 5. We have recently developed and reported on a novel, anatomically relevant, rat model of acute postoperative pain associated with inguinal hernia repair 6. Cardinal features of the model include surgery‐induced deficits in locomotor activity and activation of c‐Fos expression in the dorsal horn of the spinal cord that are reversible upon analgesic administration.
In this study, we sought to further characterize this novel model of postoperative pain with an emphasis on the affective component of pain. The affective dimension of pain includes pain‐related unpleasantness, fear, and arousal, with a strong urge to escape or avoid this unpleasantness 7. The severity of acute postoperative pain can have a significant negative impact on patients' overall well‐being, causing increased psychological distress 5, 8, 9. In patients who underwent ventral hernia repair, 38.7% reported strong affective components associated with their postoperative pain 24 months following surgery 10. The place escape/avoidance paradigm (PEAP) is a well‐validated model of pain affect and aversion in rodents 11, 12. The PEAP is used to dissociate the multidimensional pain experience in rodents by measuring the affective/motivational component of pain processing independent of the sensory component 13, 14. In this paradigm, animals demonstrate avoidance of a preferred test location (dark arena) which is associated with the application of a noxious mechanical stimulus to the injured area. The PEAP has been validated in numerous pain models including those for inflammatory 15, 16 and neuropathic 14, 17, 18 pain. However, to our knowledge, it has yet to be validated in a rodent model of postoperative pain.
Thus, the initial aim of this study was to characterize the affective component of acute postoperative pain in the novel rat model of acute postoperative pain associated with inguinal hernia repair using a modified PEAP (mPEAP), further validating it as a model of pain experience. A second aim was to investigate the ability of opioid and nonopioid analgesics to modulate the affective dimension of postoperative pain in this model. Escape/avoidance behavior in the mPEAP model is amenable to treatment with morphine when administered systemically 14, 17 and so was used herein. The nonsteroidal antiinflammatory drug (NSAID) carprofen has not previously been characterized in the PEAP but was efficacious in this model of inguinal hernia repair pain 6 and so was also used herein.
Materials and Methods
Animals
Male Lister hooded rats (200–250 g on arrival; Charles River, Margate, UK) were used in all studies and were individually housed in clear polycarbonate cages (L × W × H = 480 × 265 × 210 mm; floor area 940 cm2) containing wood shavings as bedding in a home cage monitoring system (Opto‐M3 Dual Axis System; Columbus Instruments, Columbus, OH, USA). Food and water were available ad libitum. Rats were maintained under standard laboratory conditions of temperature (21 ± 3°C), humidity (45–65%), and lighting (12:12 h light/dark cycle lights on at 08:00 h). In all experiments, the sequencing of surgery and testing was pseudo‐randomized to control for any possible confounding effects of order of testing. The experimental protocols were carried out in accordance with the guidelines and approval of the Animal Care and Research Ethics Committee, National University of Ireland, Galway, under license from the Irish Department of Health and in compliance with the European Communities Council directive 86/609 as well as the ARRIVE guidelines from the National Centre for the Replacement Refinement and Reduction of Animals in Research 19.
Surgery
The surgical procedure employed was based on the most commonly used clinical open‐surgery Lichtenstein indirect inguinal hernia repair technique 20, 21, 22 and has been described in detail previously 6. Briefly, rats were anaesthetized using isoflurane and an incision of 3 cm in length was made in the right inguinal area (located in the lower abdomen adjacent to the pubic area) through the skin, fascia, and muscle. The spermatic cord and surrounding structures were identified, excess fat removed, and the spermatic cord and surrounding area was elevated from the posterior wall of the canal with the aid of polyethylene PTFE tubing (OD 0.97 mm, ID 0.58 mm). A piece of sterile polyester textile mesh (Covidien, North Haven, CT, USA) was cut to the required size (2.5 cm length and 2 cm width) and shape and was implanted on the floor of the canal, sutured to the pubic tubercle with polypropylene 5‐0 suture (5‐0 Surgipro II; Covidien). The tails of the mesh were then crossed and sutured. The ends of the tails were then trimmed and placed into the inguinal canal. The fascia was closed by continuous suture polypropylene 5‐0 (5‐0 Surgipro II; Covidien) and the skin by continuous synthetic absorbable suture (Polysorb 5‐0; Covidien). Rats in the sham group were anaesthetized and then left under anesthesia (without any incision) for an identical duration (1 h) to the surgery groups.
Home Cage Monitoring System
The Opto‐M3 Dual Axis system (Columbus Instruments) was used to monitor horizontal and vertical locomotor activity continuously in the home cage environment (25 lux) as described previously 6. Any differences in home cage activity in this study were expected to be less than previously reported 6, as the early postoperative period occurred in the light phase as opposed to the dark phase to allow for mPEAP testing.
Open Field System
The open field system (Opto‐M3 Triple Axis System; Columbus Instruments) was identical to that described previously 6. The arenas (7 lux) were cleaned with mild detergent and dried to remove odor cues between successive rats. Open field testing was conducted at 1 h prior to mPEAP testing (2 h postsurgery) and again at 7 days postsurgery for the initial characterization study (Experiment 1) and at 1 h prior to mPEAP testing (2 h postsurgery) for the analgesic study (Experiment 2). Strong surgery‐induced deficits in locomotor activity were apparent at 2 h postsurgery in previous studies 6, and thus, this time point was chosen herein.
Place Escape/Avoidance Paradigm Testing
The place escape/avoidance paradigm (PEAP) measures and dissociates the multidimensional pain experience in rodents 12, and we used a modified version of this paradigm (mPEAP). Our arena consisted of a 64 × 31 × 32 cm wooden arena positioned on top of a wire mesh. The arena is comprised of a light side (lux 100) and a dark side with a central wooden partition with a small opening allowing unrestricted movement between the two sides during the 30‐min behavioral test. To begin, the animal is placed into the light arena, facing away from the dark chamber. When in the dark chamber, the animal receives a noxious stimulation (6 g von Frey filament applied for 5 second, or until a response is observed), to the site of incision in the inguinal area at an interval of every 15 second. No stimulation was applied when the animal was in the light chamber. This is a slight modification on the traditional PEAP protocol which is used in conjunction with paw‐directed pain models and where the contralateral paw is traditionally stimulated in the light arena. The decision not to stimulate the animals when in the light arena was taken for a number of reasons. First, previous von Frey filament studies in this model suggested that stimulation of the contralateral side of the inguinal area may have been noxious/aversive in itself and might have confounded the mPEAP results. Secondly, we reasoned that sham animals would serve as the more important control in this experiment rather than contralateral stimulation in surgery animals, as sham animals are devoid of any incision or injury and thus should display no injury‐related mechanical hypersensitivity. The 6 g von Frey filament was chosen as a noxious stimulus, based on pilot work which demonstrated that it consistently elicited a response in both sham and surgery animals. A response was defined as a jump, scratch, or biting/licking of stimulated area. The behavior of the animal was recorded using a camera placed above the light‐side chamber and time that the animal spent in the light chamber was subsequently measured. Whether or not the animal responded to the noxious stimulus was also recorded during the trial and a percentage response for each animal was subsequently calculated. 24 h prior to surgery, the animals were placed into the arena and allowed to freely explore it for 30 min without receiving any stimulation. Place escape/avoidance testing was performed at 3 h and 7 days after inguinal hernia repair surgery for the initial characterization study (Experiment 1) and at 3 h postsurgery for the analgesic study (Experiment 2).
Drugs
Carprofen (Pfizer, Dublin, Ireland) at a dose of 5 mg/kg or saline vehicle was administered subcutaneously (s.c.) in a volume of 1 mL/kg 1 h prior to surgery (4 h prior to mPEAP testing). Morphine sulfate (Antigen Pharmaceuticals, Roscrea, Ireland) or saline vehicle was administered s.c. at a dose of 3 mg/kg 2 h and 30 min postsurgery (30 min prior to mPEAP testing) in a volume of 1 mL/kg. Drug doses and times of administration were based on the pharmacokinetics of the drugs, previous work with the hernia pain model 6 as well published studies demonstrating their efficacy in animal studies modeling the affective component of pain processing 14, 17. Three rats that received morphine had to be excluded from the final analysis as they displayed stereotyped behaviors such as biting and licking of the wooden partition and wire floor. This was to such an extent that it is very likely to have distracted the rats’ attention away from the noxious von Frey stimulus, thereby resulting in a lack of response to the noxious stimulus and hence these rats were excluded from the analysis.
Experimental Protocol
Two experiments were carried out, namely an initial characterization study (Experiment 1) and an analgesic study (Experiment 2). For the initial characterization study, there were two groups: sham (n = 10) and surgery (n = 8). For the analgesic study, there were 6 treatment groups in total: sham saline (n = 8), sham morphine 3 mg/kg (n = 7), sham carprofen 5 mg/kg (n = 7), surgery saline (n = 7), surgery morphine 3 mg/kg (n = 9), and surgery carprofen 5 mg/kg (n = 7). For both experiments, rats were randomly assigned to each group. For the initial characterization experiment, animals were monitored in the home cage throughout the entire duration of the study. Open field testing was conducted at 2 h and 7 days postsurgery. mPEAP testing was performed at 3 h and 7 days postsurgery. For the analgesic study, animals were again monitored in their home cage for the entire duration of the study and were subject to open field testing at 2 h and testing in the mPEAP at 3 h postsurgery. Animals were killed by either decapitation or transcardial perfusion (heparinized saline followed by 4% paraformaldehyde in a phosphate buffered saline solution) at 8 days postsurgery for the initial characterization study and at 3 h postsurgery for the analgesic study. A male experimenter performed all surgical procedures and open field testing while a female experimenter conducted all mPEAP testing. The experimental protocol for both studies is depicted in Figure 1.
Figure 1.
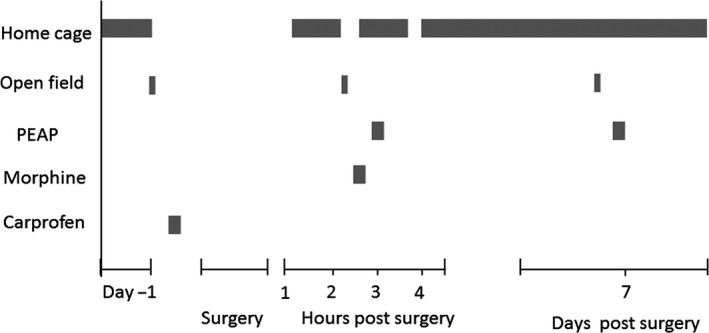
Experimental protocol for place escape/avoidance paradigm characterization experiments.
Data Analysis
Statistical analysis was performed using SPSS 20.0 software (IBM SPSS, New York, NY, USA). Data were analyzed using parametric statistics if they were found to follow a normal or approximately normal distribution within group (Shapiro–Wilk P > 0.05) and if the between‐group variances were homogeneous or approximately homogeneous (Levene's test P > 0.05). If data were found to follow a normal distribution, repeated‐measures analysis of variance (ANOVA) was performed to determine main effects of time, surgery or drug treatment, or their interactions. Fisher's least significant difference (LSD) post hoc tests or unpaired two‐tailed t‐tests with or without Bonferroni's correction were used as appropriate to determine where the differences arose between the groups and across time points. Nonparametric data were analyzed by Friedman's ANOVA by ranks or Kruskal–Wallis tests, followed by Mann–Whitney U‐test or Wilcoxon signed‐ranks test. For ease of presentation, all data are presented as mean + SEM. P < 0.05 was considered statistically significant. Results were depicted graphically with the aid of GraphPad Prism 5.0 (GraphPad Software, La Jolla, CA, USA).
Results
Experiment 1: Characterization of the Effects of Inguinal Hernia Repair Surgery in the mPEAP
Percentage Time Spent in the Light Compartment
The entire 30‐min trial duration was split into 5 min time bins, and the percentage time spent in the light compartment was calculated for each interval and subsequently plotted.
At 3 h postsurgery, there was a significant main effect of time (F 5,80 = 11.97, P < 0.001), surgery (F 1,60 = 47.74, P < 0.001) and a significant time x surgery interaction (F 1,16 = 20.53, P < 0.001) on the percentage time spent in the light compartment (Figure 2A). The surgery group spent a significantly higher percentage of time in the light compartment compared to the sham group at the 20 min, 25 min, and 30 min time points (P < 0.05), confirming a higher degree of aversion to noxious stimulation of the inguinal area in the surgery group compared with the sham group. On day 7 postsurgery, no differences were observed between the groups at any of the time points (Figure 2B).
Figure 2.
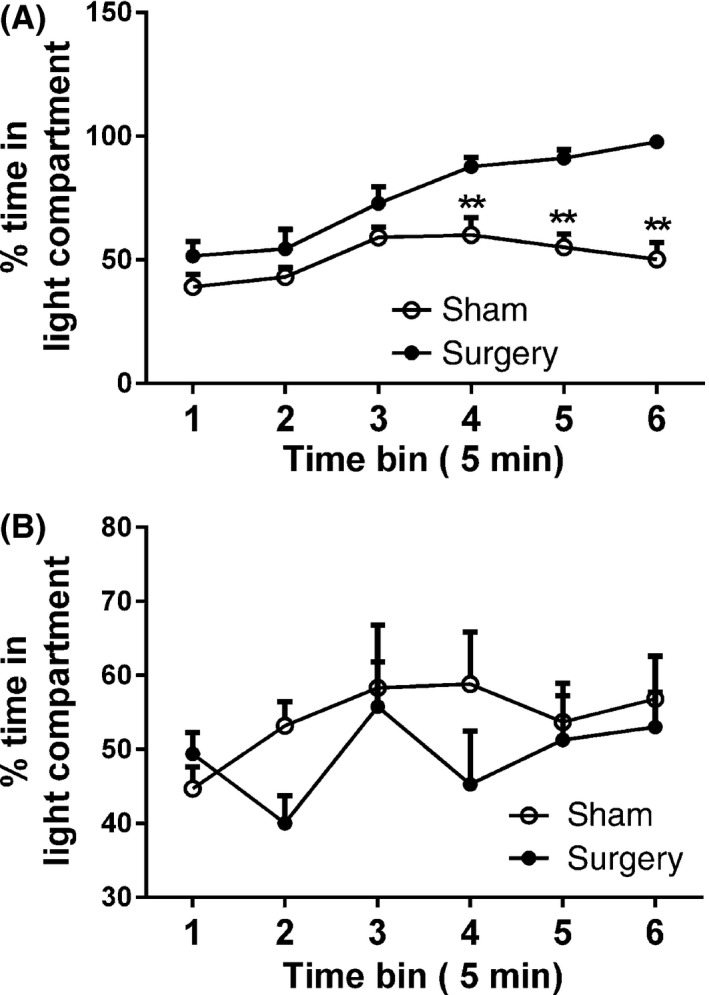
Percentage time spent in the light compartment (A) 3 h postsurgery and (B) 7 days postsurgery during the mPEAP trial. Data are mean + SEM (n = 8–10). **P < 0.01 versus sham.
Percentage Response to Noxious Stimulation
Student's unpaired two‐tailed t‐test was used to compare the percentage response to the noxious stimulus applied in the dark between the two groups over the entire 30‐min trial. At 3 h postsurgery, the surgery group had a significantly increased percentage response when compared to the sham control group (t 16 = 3.9, P < 0.01) (Figure 3A). On day 7 postsurgery, the surgery group also had a significantly increased percentage response compared to the sham control group (t 16 = 4.96, P < 0.01) (Figure 3B).
Figure 3.
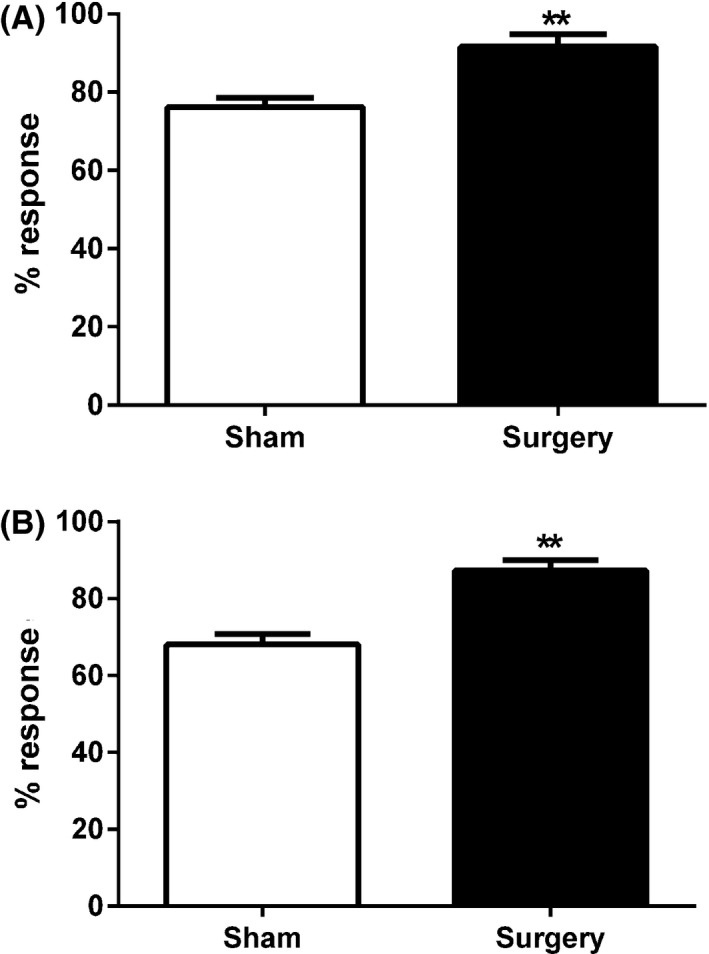
Percentage response to noxious von Frey hair stimulation during the 30‐min mPEAP trial at (A) 3 h postsurgery and (B) 7 days postsurgery. Data are mean + SEM (n = 8–10). **P < 0.01 versus sham.
Assessment of Locomotor Activity Following Surgery
Home cage activity following surgery. For the initial mPEAP characterization study, home cage activity was analyzed during the period 1–3 h postsurgery, as previous work has demonstrated that this time frame is where the peak effects of surgery on locomotor activity are most apparent 6. Analysis focused on vertical activity as it proved to be the most robust behavioral phenotype associated with the model 6.
Student's unpaired two‐tailed t‐test was used to compare home cage vertical activity during the period 1–3 h postsurgery before mPEAP testing. The surgery group displayed significantly decreased levels of vertical activity compared to the sham group (t 16 = 3.3, P < 0.01) (Figure 4).
Figure 4.
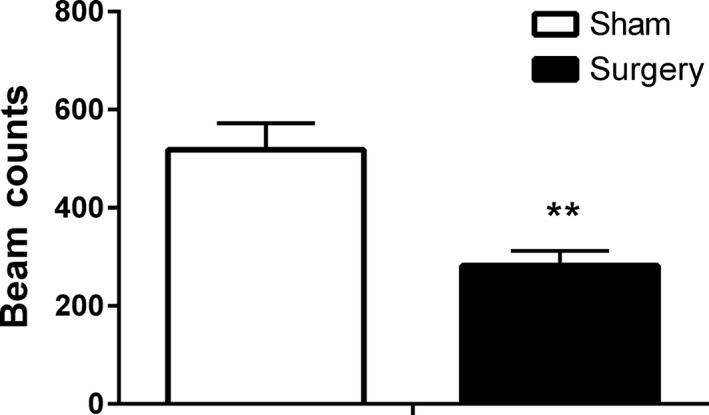
Effect of inguinal hernia repair surgery on home cage vertical activity during the period 1–3 h postsurgery. Data are mean + SEM (n = 8–10). **P < 0.01 sham versus surgery.
Open field activity following surgery. Animals underwent open field testing before each of the mPEAP trials in order to confirm that the previously characterized behavioral phenotype associated with this model, surgery‐induced deficits in open field locomotor activity, were apparent in this study. Similar to home cage activity, analysis focused on vertical activity.
Repeated‐measures ANOVA revealed a significant effect of time (F 1,16 = 56.91, P < 0.001), surgery (F 1,16 = 4.41, P < 0.05), and a time x treatment interaction (F 1,16 = 11.71, P < 0.05) on open field vertical activity following surgery. Post hoc analysis revealed that the behavioral phenotype associated with the model was apparent at 2 h postsurgery, with the surgery group displaying significantly decreased levels of activity compared to the sham group (P < 0.01). There was no difference between the groups at 7 days postsurgery (Figure 5).
Figure 5.
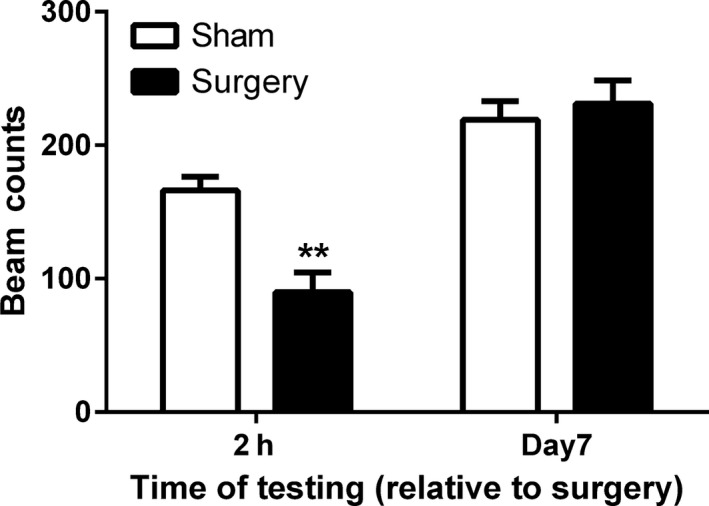
Effect of inguinal hernia repair surgery on vertical activity in the open field at 2 h or 7 days following surgery. Data are mean + SEM (n = 7–9). **P < 0.01 sham versus surgery.
Experiment 2: Effect of Morphine and Carprofen on mPEAP Testing Following Surgery
Percentage Time Spent in the Light Compartment
The entire 30‐min trial was split into 5 min bins, and the percentage time spent in the light compartment was calculated for each time bin and subsequently plotted.
Two‐way repeated‐measures ANOVA with surgery and drug as factors revealed a significant effect of time (F 5,195 = 10.55, P < 0.001) and a time x drug treatment interaction (F 10,195 = 2.41, P < 0.05) but no main effects of surgery (F 1,39 = 1.93, P > 0.05) or drug treatment (F 2,39 = 1.55, P > 0.05) on the percentage time spent in the light compartment (Figure 6). Post hoc analysis revealed that the surgery saline group spent a significantly greater percentage of time in the light compartment compared to the sham saline group at the 4th and 6th time bins (P < 0.05). Percentage time in the light compartment was significantly lower in the surgery morphine group compared to the surgery saline group at the 4th time bin (P < 0.01). Like morphine, carprofen also attenuated surgery‐induced increases in percentage time spent in the light compartment at the 4th (P < 0.01) and 6th (P < 0.05) time bins where the surgery carprofen group exhibited significantly lower percentage time in the light compartment compared to the surgery saline group. There was no significant effect of drug treatment on time in the light compartment for sham animals (Figure 6).
Figure 6.
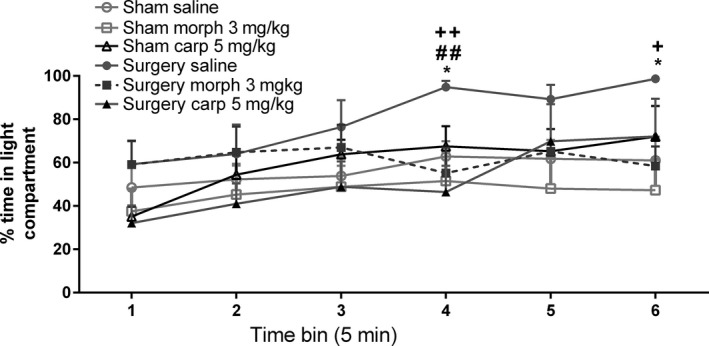
Effect of morphine (morph) and carprofen (carp) on the percentage time spent in the light compartment during the mPEAP trial in sham and surgery rats. Data are mean + SEM (n = 7–9). *P < 0.05 sham saline versus surgery saline; ## P < 0.01 surgery morph versus surgery saline; + P < 0.05, ++ P < 0.01 surgery carprofen versus surgery saline.
Percentage Response to Noxious Stimulation
Two‐way ANOVA revealed a significant effect of surgery (F 1,41 = 29.93, P < 0.05) and drug treatment (F 2,41 = 5.49, P < 0.05) on the percentage response to noxious stimulation during the mPEAP trial. Post hoc analysis revealed that the surgery saline group displayed an increased percentage response compared to the sham saline group (P < 0.01). Morphine reduced the percentage response to noxious stimulation irrespective of surgery, with both the sham morphine group and the surgery morphine group displaying a decreased percentage response, compared to respective saline‐treated controls (P < 0.01) (Figure 7).
Figure 7.
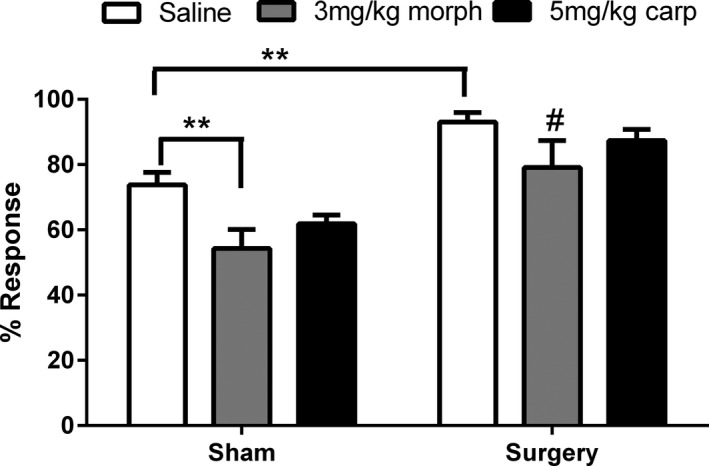
Effect of morphine and carprofen on the percentage response to noxious von Frey hair stimulation following surgery during mPEAP trial. Data are mean + SEM (n = 7–9). **P < 0.01 versus sham saline # P < 0.05 versus surgery saline.
Assessment of Home Cage and Open Field Locomotor Activity Following Surgery
For the mPEAP analgesic study (Experiment 2), home cage activity was analyzed for the period 1–3 h postsurgery only, owing to the short duration of the postsurgical period in this experiment, as well as being the period where surgery‐induced deficits in locomotor activity are most apparent. Analysis focused on vertical activity.
A Kruskal–Wallis test revealed an overall significant effect between all groups (K = 22.33, P < 0.001) on home cage vertical activity during the period 1–3 h postsurgery. Mann–Whitney U‐tests revealed that the surgery saline group displayed significantly reduced activity compared to the sham saline group (P < 0.01), while the surgery 5 mg/kg carprofen group displayed significantly increased activity compared to the surgery saline group (P < 0.01), indicating an attenuation of the surgery‐induced locomotor deficits (Figure 8). Morphine had no significant effects in sham or surgery animals which was not unexpected given that it was administered 2.5 h postsurgery.
Figure 8.
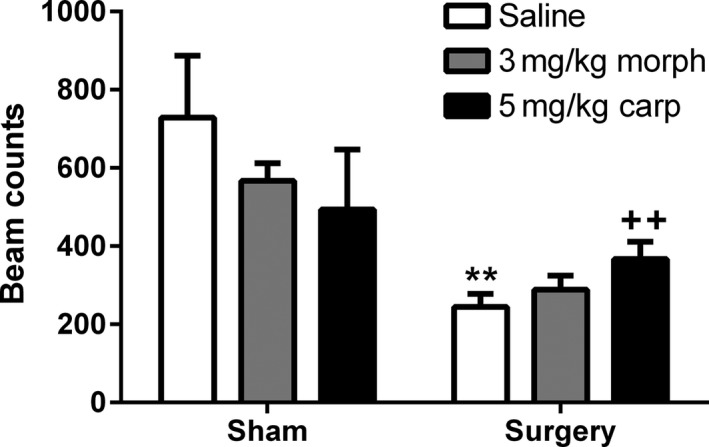
Effect of morphine and carprofen in sham and surgery animals on home cage vertical activity during the period 1–3 h postsurgery. Data are mean + SEM (n = 7–9) **P < 0.01 versus sham saline ++ P < 0.01 versus surgery saline.
Surgery‐induced reductions in open field activity were observed as for Exp 1, but carprofen had no effect and morphine was administered after open field testing (data not shown).
Discussion
This manuscript reports the behavioral and pharmacological characterization of the affective dimension of postoperative pain following inguinal hernia repair using the mPEAP. In the mPEAP, the aversive/unpleasant nature of pain is measured by the avoidance of a preferred location (dark chamber) and an increase in the amount of time spent in the aversive (light) chamber reflects the degree of aversion to noxious stimulation 12. At 3 h postsurgery, animals displayed a significantly increased percentage of time in the light compartment, choosing to reside in the aversive light chamber within 20 min of test onset and spending nearly 100% of their time in that compartment by the end of the trial. These results correlate strongly with the results obtained with other rodent models of pain in the PEAP 12, 14. Systemic administration of either morphine or carprofen decreased the time spent in the light arena at 3 h postsurgery at discrete time points during the 30‐min trial. The percentage response to noxious stimulation was significantly increased in surgery animals compared to sham controls at 3 h postsurgery and also at 7 days postsurgery. However, there was no difference in time spent in the light chamber between the two groups at day 7, suggesting that the sensory‐discriminative response to noxious stimulation persisted up to 7 days postsurgery, while the affective/motivational dimension did not.
Three major nerves innervate the inguinal area: the ilioinguinal, iliohypogastric, and genitofemoral; therefore, it is possible that the inguinal area is more sensitive to mechanical stimulation than the plantar surface of the hind paw in the absence of any intervention, making sham animals more likely to respond to any stimulation, noxious, or nonnoxious. This may explain the high response in sham animals at both 3 h and 7 days postsurgery, approximately 70% in both cases. Previous PEAP studies have reported a lower response to stimulation of uninjured hind paw with the use of a noxious von Frey stimulus 15, 16, 23, but to our knowledge, this is the first study to investigate stimulation of the inguinal area. Indeed, the decision not to stimulate the contralateral side in the mPEAP was taken with the above reason in mind. The lack of avoidance of the stimulus observed in surgery animals at 7 days postsurgery could be because the stimulus may not have been of sufficient intensity to drive the animals into the aversive light context after such a long postsurgery recovery period, that is, at 7 days postsurgery, test animals found the 6 g von Frey stimulus less unpleasant, or they were better able to tolerate it than at 3 h postsurgery, where active avoidance was observed. In addition, it could be explained by the observation that animals that undergo surgery display acute pain‐related anxiety in the early postoperative period 24, 25. In the case of the mPEAP, such pain‐related fear avoidance may exacerbate a painful response to a noxious stimulus in the early hours following surgery and hence the active avoidance of the stimulus. However, by day 7, the pain‐related fear avoidance may have dissipated and active avoidance is not observed suggesting no anxiety‐induced exacerbation of pain. Exacerbation of pain by anxiety has been well documented in both the clinical 26, 27 and preclinical 28, 29 literatures.
Our results demonstrate that both morphine and carprofen were efficacious during the mPEAP trial, significantly decreasing the time spent in the light side of the arena at discrete time points during the 3 h postsurgery test period. These data indicate the ability of these analgesics to attenuate the affective component of acute postoperative pain in this model. These results are in agreement with previous reports which have demonstrated attenuated escape/avoidance behavior with morphine 14, 30. Morphine has traditionally been the gold standard for postoperative analgesia and is often used as a comparator with which putative analgesics are assessed 31, 32. No overt effects of drug treatment were observed on mPEAP performance in sham animals. Thus, although time spent in the light compartment was not completely attenuated by either drug, both do modulate affective behavioral responding in this model at discrete time points and further characterize it as a novel model of acute postoperative pain with both somatic and affective pain dimensions. Morphine also reduced the percentage response to noxious stimulation in both sham and surgery animals compared to corresponding controls, suggesting that the 3 mg/kg dose was effective in relieving both the sensory and affective dimensions of pain. Carprofen failed to attenuate mechanical hyperalgesia but did reduce time spent in the light area in the mPEAP. This dissociation of the analgesic effects of carprofen on somatic and affective dimensions of pain has been observed previously in the mPEAP with another NSAID, aspirin 15. Indeed, an attenuation of the affective/motivational component of pain has consistently been observed at lower doses of analgesics than that required for mechanical hypersensitivity in the mPEAP 13, 14, 18, 30, suggesting that the paradigm is primarily sensitive to the affective dimension of pain.
The inguinal hernia repair model of acute postoperative pain produced a robust and reproducible behavioral response in the mPEAP, as evidenced by an increased time spent in the light compartment in surgery animals in both experiment 1 and experiment 2. When the magnitude and duration of effect in the mPEAP for this model of acute pain are compared to more chronic neuropathic and inflammatory models, a number of differences are apparent. In a model of L5 spinal nerve ligation (SNL), differences in duration spent in the light arena between sham and SNL groups emerged much sooner, after only 5 min into the test and persisted for the entire 30‐min trial duration 12. This is in contrast to the model reported here, where differences only emerged between the sham and surgery groups 20 min into the trial. Similar effects to that seen with SNL have also been reported in other neuropathic 14, 18 and chronic inflammatory pain models 12, 15. All of these models report an earlier emergence and greater magnitude of difference (particularly in the early part of the trial) between sham and injured groups compared to the model of inguinal hernia repair pain reported herein. However, it should be noted that by the end of the mPEAP trial, inguinal hernia repair animals were spending 100% of their time in the light compartment, similar to those following neuropathic and inflammatory procedures.
Locomotor activity following surgery was also assessed to confirm the presence of the surgery‐induced deficits in activity which is a hallmark of this model 6. For the present study, animals were placed on a traditional light–dark cycle (lights on at 08:00 h) in their home cage as this was more compatible with mPEAP testing which was performed at 100 lux and during the animals’ light phase. This contrasts with our previous report on the model 6 where animals were kept on a reverse light–dark cycle to provoke a high baseline of locomotor activity in the early postoperative period, thereby giving a bigger window over which to see Surgery‐induced locomotor deficits. Open field testing was carried out as described previously and performed under dim light (7 lux). The surgery‐induced deficits in both home cage and open field vertical locomotor activity observed previously 6 were again evident in the present study, confirming that this phenotype is retained on a normal light:dark cycle. The surgery‐induced reduction in home cage activity was attenuated by carprofen, corroborating our previous results 6 and confirming that the surgery‐induced locomotor impairment is pain‐related.
The PEAP was developed to help dissociate the complex and multidimensional nature of pain and to address limitations associated with the use of traditional tests for sensory hypersensitivity. The major benefit of the paradigm is that it requires no pretraining and measures drug effects on the acquisition of behavior 13. However, the paradigm is not without caveats, an important one being that a noxious stimulus still has to be applied to the injured area to initiate the affective conditioning of the pain response 14. As a result, it is not possible to conclusively rule out that analgesic‐induced manipulation of mPEAP behavior is not solely due to effects on the sensory dimension of the pain experience—unless the analgesic specifically affects the time spent in the light compartment without affecting the sensory hypersensitivity response, as was the case for carprofen herein. In conclusion, the novel findings described here expand upon the characterization of this model of acute postoperative pain to include an affective dimension. An affective dimension of postoperative pain was previously demonstrated using a conditioned place preference paradigm in the hind paw incision model 33. However, the present study demonstrates, for the first time, an affective dimension of acute postoperative pain in rodents using the mPEAP. With a significant proportion of patients experiencing moderate‐to‐severe postoperative pain up to 7 days postsurgery that negatively affects their physical and mental well‐being 5, this study may help to better understand the mechanisms underlying the multidimensional nature of acute postoperative pain.
Conflict of Interest
D.C.B. and O.M. were employees of Covidien, a commercial sponsor of the study. The other authors declare no conflict of interest.
Acknowledgment
This project was funded by a grant from the Irish Industrial Development Agency in partnership with Covidien.
The first two authors contributed equally to this work.
References
- 1. Dolin SJ, Cashman JN, Bland JM. Effectiveness of acute postoperative pain management: I. Evidence from published data. Br J Anaesth 2002;89:409–423. [PubMed] [Google Scholar]
- 2. Jenkins JT, O'Dwyer PJ. Inguinal hernias. BMJ 2008;336:269–272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Massaron S, Bona S, Fumagalli U, Battafarano F, Elmore U, Rosati R. Analysis of post‐surgical pain after inguinal hernia repair: A prospective study of 1,440 operations. Hernia 2007;11:517–525. [DOI] [PubMed] [Google Scholar]
- 4. McGrath B, Elgendy H, Chung F, Kamming D, Curti B, King S. Thirty percent of patients have moderate to severe pain 24 hr after ambulatory surgery: A survey of 5,703 patients. Can J Anaesth 2004;51:886–891. [DOI] [PubMed] [Google Scholar]
- 5. Taylor RS, Ullrich K, Regan S, Broussard C, Schwenkglenks M, Taylor RJ, et al. The impact of early postoperative pain on health‐related quality of life. Pain Pract 2013;13:515–523. [DOI] [PubMed] [Google Scholar]
- 6. Bree D, Moriarty O, O'Mahony CM, Morris B, Bannerton K, Broom DC, et al. Development and characterization of a novel, anatomically relevant rat model of acute postoperative pain. J Pain 2015;16:421–435 e6. [DOI] [PubMed] [Google Scholar]
- 7. Melzack R, Casey KL. Sensory, motivational, and central control determinants of pain: A new conceptual model In: Kenshalo D, editor. The Skin Senses. Springfield, IL: Thomas CC, 1968;423–443. [Google Scholar]
- 8. Ali M, Winter DC, Hanly AM, O'Hagan C, Keaveny J, Broe P. Prospective, randomized, controlled trial of thoracic epidural or patient‐controlled opiate analgesia on perioperative quality of life. Br J Anaesth 2010;104:292–297. [DOI] [PubMed] [Google Scholar]
- 9. Levy BF, Scott MJ, Fawcett W, Fry C, Rockall TA. Randomized clinical trial of epidural, spinal or patient‐controlled analgesia for patients undergoing laparoscopic colorectal surgery. Br J Surg 2011;98:1068–1078. [DOI] [PubMed] [Google Scholar]
- 10. Gronnier C, Wattier JM, Favre H, Piessen G, Mariette C. Risk factors for chronic pain after open ventral hernia repair by underlay mesh placement. World J Surg 2012;36:1548–1554. [DOI] [PubMed] [Google Scholar]
- 11. Sufka KJ. Conditioned place preference paradigm: A novel approach for analgesic drug assessment against chronic pain. Pain 1994;58:355–366. [DOI] [PubMed] [Google Scholar]
- 12. LaBuda CJ, Fuchs PN. A behavioral test paradigm to measure the aversive quality of inflammatory and neuropathic pain in rats. Exp Neurol 2000;163:490–494. [DOI] [PubMed] [Google Scholar]
- 13. Boyce‐Rustay JM, Zhong C, Kohnken R, Baker SJ, Simler GH, Wensink EJ, et al. Comparison of mechanical allodynia and the affective component of inflammatory pain in rats. Neuropharmacology 2010;58:537–543. [DOI] [PubMed] [Google Scholar]
- 14. Pedersen LH, Blackburn‐Munro G. Pharmacological characterisation of place escape/avoidance behaviour in the rat chronic constriction injury model of neuropathic pain. Psychopharmacology 2006;185:208–217. [DOI] [PubMed] [Google Scholar]
- 15. LaBuda CJ, Fuchs PN. Low dose aspirin attenuates escape/avoidance behavior, but does not reduce mechanical hyperalgesia in a rodent model of inflammatory pain. Neurosci Lett 2001;304:137–140. [DOI] [PubMed] [Google Scholar]
- 16. Uhelski ML, Fuchs PN. Naltrexone fails to increase pain affect in response to inflammatory pain in a novel escape/avoidance paradigm. Physiol Behav 2009;98:263–267. [DOI] [PubMed] [Google Scholar]
- 17. LaBuda CJ, Fuchs PN. Morphine and gabapentin decrease mechanical hyperalgesia and escape/avoidance behavior in a rat model of neuropathic pain. Neurosci Lett 2000;290:137–140. [DOI] [PubMed] [Google Scholar]
- 18. Baastrup C, Jensen TS, Finnerup NB. Pregabalin attenuates place escape/avoidance behavior in a rat model of spinal cord injury. Brain Res 2011;1370:129–135. [DOI] [PubMed] [Google Scholar]
- 19. Kilkenny C, Browne WJ, Cuthill IC, Emerson M, Altman DG. Improving bioscience research reporting: The ARRIVE guidelines for reporting animal research. PLoS Biol 2010;8:e1000412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Amid PK, Shulman AG, Lichtenstein IL. The Lichtenstein open “tension‐free” mesh repair of inguinal hernias. Surg Today 1995;25:619–625. [DOI] [PubMed] [Google Scholar]
- 21. Amid PK. The Lichtenstein repair in 2002: An overview of causes of recurrence after Lichtenstein tension‐free hernioplasty. Hernia 2003;7:13–16. [DOI] [PubMed] [Google Scholar]
- 22. Lichtenstein IL, Shulman AG, Amid PK, Willis PA. Hernia repair with polypropylene mesh. An improved method. AORN J 1990;52:559–565. [DOI] [PubMed] [Google Scholar]
- 23. LaBuda CJ, Fuchs PN. Attenuation of negative pain affect produced by unilateral spinal nerve injury in the rat following anterior cingulate cortex activation. Neuroscience 2005;136:311–322. [DOI] [PubMed] [Google Scholar]
- 24. Li CQ, Zhang JW, Dai RP, Wang J, Luo XG, Zhou XF. Surgical incision induces anxiety‐like behavior and amygdala sensitization: Effects of morphine and gabapentin. Pain Res Treat 2010;2010:705874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Roeska K, Doods H, Arndt K, Treede RD, Ceci A. Anxiety‐like behaviour in rats with mononeuropathy is reduced by the analgesic drugs morphine and gabapentin. Pain 2008;139:349–357. [DOI] [PubMed] [Google Scholar]
- 26. Ploghaus A, Narain C, Beckmann CF, Clare S, Bantick S, Wise R, et al. Exacerbation of pain by anxiety is associated with activity in a hippocampal network. J Neurosci 2001;21:9896–9903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Asmundson GJ, Taylor S. Role of anxiety sensitivity in pain‐related fear and avoidance. J Behav Med 1996;19:577–586. [DOI] [PubMed] [Google Scholar]
- 28. Greenwood‐Van Meerveld B, Gibson M, Gunter W, Shepard J, Foreman R, Myers D. Stereotaxic delivery of corticosterone to the amygdala modulates colonic sensitivity in rats. Brain Res 2001;893:135–142. [DOI] [PubMed] [Google Scholar]
- 29. Roeska K, Ceci A, Treede RD, Doods H. Effect of high trait anxiety on mechanical hypersensitivity in male rats. Neurosci Lett 2009;464:160–164. [DOI] [PubMed] [Google Scholar]
- 30. LaGraize SC, Borzan J, Peng YB, Fuchs PN. Selective regulation of pain affect following activation of the opioid anterior cingulate cortex system. Exp Neurol 2006;197:22–30. [DOI] [PubMed] [Google Scholar]
- 31. McEvoy A, Livingstone JI, Cahill CJ. Comparison of diclofenac sodium and morphine sulphate for postoperative analgesia after day case inguinal hernia surgery. Ann R Coll Surg Engl 1996;78:363–366. [PMC free article] [PubMed] [Google Scholar]
- 32. Pettersson N, Berggren P, Larsson M, Westman B, Hahn RG. Pain relief by wound infiltration with bupivacaine or high‐dose ropivacaine after inguinal hernia repair. Reg Anesth Pain Med 1999;24:569–575. [DOI] [PubMed] [Google Scholar]
- 33. Navratilova E, Xie JY, Okun A, Qu C, Eyde N, Ci S, et al. Pain relief produces negative reinforcement through activation of mesolimbic reward‐valuation circuitry. Proc Natl Acad Sci USA 2012;109:20709–20713. [DOI] [PMC free article] [PubMed] [Google Scholar]


