Summary
Aims
Chemical entities containing mercapto group have been increasingly attractive in the therapy of central nerve system (CNS) diseases. In the recent study, we screened a series of mercapto‐tacrine derivatives with synergistic neuropharmacological profiles in vitro.
Methods
We investigated the effect and mechanism of ST09, a thioester derivative of tacrine containing a potential mercapto group, on the vascular dementia (VaD) model of rat induced by bilateral common carotid arteries occlusion (2‐VO).
Results
ST09 and its active metabolite ST10 retained excellent inhibition on acetylcholinesterase (AChE) activity. ST09 significantly attenuated the 2‐VO‐induced impairment in spatial acquisition performance and inhibited the 2‐VO‐induced rise of AChE activity. In the VaD model, ST09 attenuated the oxidative stress and decreased the apoptosis in the cortex and hippocampus. Compared with donepezil, ST09 exhibited a better effect on the regeneration of free thiols in 2‐VO rats. Interestingly, ST09, not donepezil, greatly improved glucose metabolism in various brain regions of 2‐VO rats using functional imaging of 18F‐labeled fluoro‐deoxyglucose (FDG) positron emission tomography (PET).
Conclusions
ST09 may serve as a more promising agent for the therapy of VaD than tacrine owing to the introduction of a potential mercapto group into the parent skeleton.
Keywords: Acetylcholinesterase, Glucose Metabolism, Tacrine, Thioester Derivative, Vascular Dementia
Introduction
Vascular dementia (VaD) is considered as a second most common cause of dementia after Alzheimer's dementia (AD) 1. The cognitive impairment of VaD is mainly attributed to cerebrovascular pathology 2. After cerebral hypoperfusion, a series of factors, including metabolism impairment, excitotoxicity, oxidative stress, and cholinergic deficit, are involved in the progression of secondary cognitive impairment 3, 4. Although some drugs have been approved as moderate therapy for VaD 2, 5, 6, 7, 8, 9, none of the clinic drugs show a significant therapeutic effect on VaD yet. By recognizing the multifactorial nature of VaD, single chemical entity with the ability to modulate multiple targets may offer a promising approach for the treatment of dementia.
Tacrine, the first AChEI approved by the Food and Drug Administration (FDA) for Alzheimer's disease (AD), exhibits a convincing clinical effect on the cognitive function of dementia patients. However, the serious hepatotoxicity of tacrine limits its clinical use 5, 6, 7. Some chemical entities containing mercapto group, such as tiopronin, are clinically applied to treat the liver injury caused by chemical insults 8. Thus, tacrine derivates cooperated with additional mercapto fragment might be favorable to reduce its toxicity. Interestingly, chemical entities containing mercapto group, such as dithiothreitol (DTT) 9, glutathione (GSH) 10, N‐acetyl cysteine (NAC) 11, and pyritinol 12, have been increasingly attractive to the therapy of central nervous system (CNS) diseases because of their multiple pharmacological actions 13, 14, 15. Previous studies in our group and other laboratories have demonstrated that mercaptans increase long‐term potentiation (LTP) of synaptic transmission in the hippocampus10, 16, 17. Thus, the introduction of a potential mercapto group into tacrine skeleton may serve as a promising strategy for the development of novel anti‐VaD agents 18.
Recently, we developed a series of mercapto‐tacrine derivatives with the aim of improving pharmacological efficacy and overcoming the hepatotoxicity 19. We hypothesized that mercapto‐tacrine derivatives would be suitable for the therapy of VaD. Considering the unstability of mercapto‐tacrine derivatives, we further synthesized a novel thioester derivative of 6‐chloro‐tacrine, ST09, which can be transformed into mercaptan derivative in vivo. To disclose the proposed multitarget profile of ST09, in the present study, a number of in vitro and in vivo assays were performed to explore the potential anti‐VaD effect and the underlying mechanisms in the bilateral common carotid arteries occlusion (2‐VO)‐induced VaD rat model.
Materials and Methods
Animals
Adult male Sprague Dawley (SD) rats weighing 220–250 g (8–9 weeks) were obtained from the Experimental Animals of Tongji Medical College, Huazhong University of Science and Technology. The rats were housed individually on a controlled 12‐h:12‐h light–dark cycle at a constant temperature (22 ± 1°C) with free access to water and food, and allowed to acclimate a week. The use of animals for experimental procedures was conducted in accordance with the Guide for Care and Use of Laboratory Animals as adopted and promulgated by the National Institutes of Health. The experimental procedures were approved by the Animal Welfare Committee of Huazhong University of Science & Technology.
Surgery
The rats were anesthetized with pentobarbital sodium (i.p., 3%, 0.2 mL/100 g) during surgical procedure by 2‐VO. Briefly, through a midline cervical incision, both common carotid arteries were exposed and gently separated from carotid sheath and vagus nerve. The artery of each rat randomly assigned to the model groups was ligated with a 4/0 silk suture. The sham‐operated control animals were subjected to the same operation but without ligation. The temperature was maintained at 37°C during surgical procedure with the help of heating lamp. Following the surgical operation, the animals were kept in cages with food and water ad libitum.
Metabolism Studies in vitro
See details in the Supplementary methods section.
Measurement of Intracellular ROS
See details in the Supplementary methods section 20.
Biochemical Analysis
Cholinesterase activity and SH groups level were measured, respectively, by the methods of Ellman 21, Sedlak, and Lindsay 22. The details were described in the Supplementary methods section.
Histopathological Observation
See details in the Supplementary methods section.
Electron Spin Resonance (Esr) Measurement
N‐tert‐butyl‐α‐phenylnitrone (PBN) was used as a trapper of free radical 23. ESR signals were detected with an e‐scan ESR spectrometer (Burker, Karlsruhe, Germany) as described in our previous study 24. The details were described in the Supplementary methods section.
Morris Water Maze (MWM)
The spatial learning performance of rat was evaluated by MWM, as described in our previous studies 25. The details were described in the Supplementary methods section.
Western Blotting
See details in the Supplementary methods section.
18F‐labeled Fluoro‐Deoxyglucose Positron Emission Tomography (18F‐FDG‐PET)
A subset of rats tested in the MWM were scanned for cerebral uptake of 18F‐FDG under pentobarbital sodium sedation in Trans‐PET BioCaliburn LH (Raycan Technology Co., Ltd, Suzhou, China), which was a new generation commercial small‐animal PET system 26, 27. The details were described in the Supplementary methods section.
Statistical Analysis
Data from experiments were analyzed with the statistical program SPSS 18.0 software (SPSS, Chicago, IL, USA). Comparison between two groups was evaluated by an unpaired and two‐sided Student's t‐test. Differences between multiple groups were analyzed by one‐way analysis of variance (ANOVA) followed by post hoc tests (Fisher's LSD). Data are presented as mean ± SEM. Differences between experimental conditions were considered statistically significant when P < 0.05. Escape latency in Morris water maze test was analyzed by two‐way analysis of variance (ANOVA) for repeated measures followed by Turkey post hoc test.
Results
ST09 is Transformed into Mercaptan in vitro and in vivo
We first confirmed that ST09 could be transformed into mercaptan in vitro in rat blood using LC‐MS/MS method. As shown in Figure 1B and C, the retention times for ST09 and ST10 under the optimal gradient condition were 5.67 min and 5.14 min, respectively, with an overall chromatographic run time of 10 min. ST09 remained stable in saline at 37°C, but the peak area value of ST09 was decreased rapidly by 83.03 ± 0.15% after 5‐min incubation and by 96.60 ± 0.01% after 30‐min incubation in rat blood (Table S1). As expected, the peak area of ST10 (m/z 420.1→233.0) and the deacetylated metabolite of ST09 (m/z 462.0→386.0) increased by 61.8 folds after 30‐min incubation in rat blood (Table S1). After incubation, the peak area ratio of ST10/ST09 significantly increased to 29.51 ± 1.11 in blood compared with that of 1.04 × 10−2±3.72 × 10−5 in saline (Figure 1F, n = 6). Therefore, ST09 can be rapidly metabolized into ST10 by deacetylases in rat blood. In vivo, after i.p. of ST09 (5 mg/kg), the transformation from ST09 to ST10 was shown in Table S2 (n = 6). The peak area ratio of ST10/ST09 increased to 7.28 ± 0.96 by 40 min (Figure 1D–E,G) and to 15.68 ± 1.01 at 1 h (Table S2). The precise mechanisms for the transformation from ST09 to ST10 required further investigation. There are many hydrolases in the blood that can transform thioester into free thiol, such as esterase, thioesterase, and carboxylesterases, and a possible deacetylation pathway was shown in Figure 1H.
Figure 1.
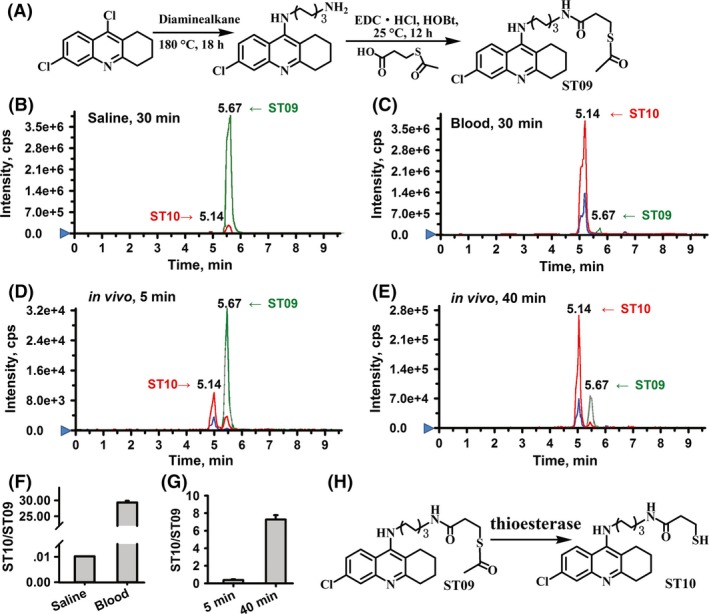
The stability of ST09 in vitro and in vivo. (A) Synthesis of ST09. Representative extracted ion chromatograms (EICs) obtained from ST09 samples in vitro incubated with saline (B) and rat blood (C) for 30 min, in vivo administrated rats with ST09 (5 mg/kg, i.p.) at 5 min (D) and at 40 min (E). (ST09: m/z 462.0→386.0; ST10: m/z 420.1→233.0). (F) The peak area ratio of ST10/ST09 after 30‐min incubation in saline and rat blood (n = 6). (G) The peak area ratio of ST10/ST09 in blood of rats at 5 min and 40 min after i.p. injection of ST09 (5 mg/kg, n = 6). Data are presented as mean ± SEM. (H) A possible metabolic pathway of ST09 in vivo.
ST09 Shows Little Hepatotoxicity in vitro and in vivo
Then, we observed whether the introduction of a potential mercapto group reduces the hepatotoxicity of tacrine. In vitro, consistent with previous study, tacrine dose dependently decreased the viability of HepG2 cells, while ST09 exhibited little effect (Figure S1A–B) on the viability of HepG2 cells. Tacrine (0.1–100 μM) increased the intracellular level of ROS in a concentration‐dependent manner (10 μM: 113.78 ± 1.84%, 100 μM: 123.16 ± 1.33%, n = 5, P < 0.01 vs vehicle; Figure S1C) and depleted the intracellular GSH in HepG2 cells, while ST09 (0.1–100 μM) did not alter intracellular ROS level but increased intracellular GSH level at 1–100 μM (1 μM: 122.85 ± 3.91%, 10 μM: 131.49 ± 2.03%, 100 μM: 114.00 ± 0.65%, n = 5, P < 0.01 vs. vehicle; Figure S1E–F). In vivo, ST09 (3.46 mg/100 g/day, 6 weeks, n = 8) did not affect the serum glutamic pyruvic transaminase (GPT)/glutamic oxaloacetic transaminase (GOT) ratio. ST09 produced little histomorphological changes in the liver of 2‐VO rats (Figure S1G). These results indicate that the introduction of a potential mercapto group into its parent skeleton significantly reduces hepatotoxicity.
ST09 Improves the Spatial Learning and Memory of VaD Rats
We investigated the effect of ST09 on learning and memory of 2‐VO‐induced VaD rat model. The safety and efficacy of AChEIs, such as donepezil, are widely demonstrated in some large clinical trials of VaD 27, 28. Thus, donepezil, an AChEI for AD and VaD therapy, was used as a positive control (0.30 mg/kg/day, i.p., 6 weeks, n = 9). In our preliminary experiment, we have measured the LD50 of different mercapto‐tacrine derivatives in rats. The LD50 of ST09 was 70 mg/kg. Thus, the dose of ST09 in the therapy of VaD was set at 1.73, 3.46, and 6.92 mg/kg, respectively. Drug intervention was started at 8th day after surgery operation and continued until the end of experiment (i.e., 49th day). From the 43rd day, animals were exposed in MWM tests to investigate the effect of ST09 on spatial learning and memory. In the visible platform trials, there were no differences in escape latency (Figure 2A) and swimming speed (Figure 2B) among the groups, suggesting that both 2‐VO operation and drug treatment did not influence the motor behavior of rats. In the spatial navigation test, from day 1 to day 4, the escape latencies decreased significantly across the 4 days of training [day: F(3,141) = 82.902, P < 0.01]. There were significant differences between the six groups from day 2 to day 4, except for day 1 [day 1: F(5,47) = 0.576, P = 0.718; day 2: F(5,47) = 4.967, P = 0.001; day 3: F(5,47) = 6.670, P < 0.001; day 4: F(5,47) = 11.228, P < 0.001]. Training day interacted significantly with rat group [day × group: F(15,141) = 1.827, P = 0.036]. A escape latency of 2‐VO group was longer than that of sham group (F(1,16) = 25.251, P < 0.01). After administration of drugs for 6 weeks, as shown in Figure 2C, the escape latency in ST09‐treated group decreased significantly than that of 2‐VO group (F(1,25) = 12.845, P < 0.001). The latencies in groups at middle and high dosage of ST09 showed much significant decrease than that of 2‐VO group (3.46 mg/kg: day 3: P < 0.01, day 4: P < 0.01; 6.92 mg/kg: day 3: P < 0.05, day 4: P < 0.01 vs. 2‐VO, n = 9), yet that in the group at low dosage was not different from that of 2‐VO group. In the spatial probe test, swimming speeds among the groups were similar (Figure 2D), indicating that the operation and drugs administration did not influence the locomotive and visual ability of rats. As shown in Figure 2E–F, the rats of 2‐VO group spent less time and travelled less distance in the target region (time: 13.11 ± 1.30 second; distance: 208.02 ± 21.52 cm, P < 0.05 vs. sham) when compared with sham group (time: 17.96 ± 1.52 second; distance: 286.22 ± 16.72 cm). ST09 at middle (3.46 mg/kg) and high (6.92 mg/kg) dosages significantly increased the duration and travelled distance in the target region compared with 2‐VO group (3.46 mg/kg: 18.14 ± 1.37 second, 286.56 ± 18.21 cm; 6.92 mg/kg: 18.45 ± 1.08 second, 273.18 ± 23.00 cm; P < 0.05 or 0.01 vs. 2‐VO; Figure 2E–F). Figure 2G shows the swimming traces of different groups in the spatial probe test uniformly distributed around four zones.
Figure 2.
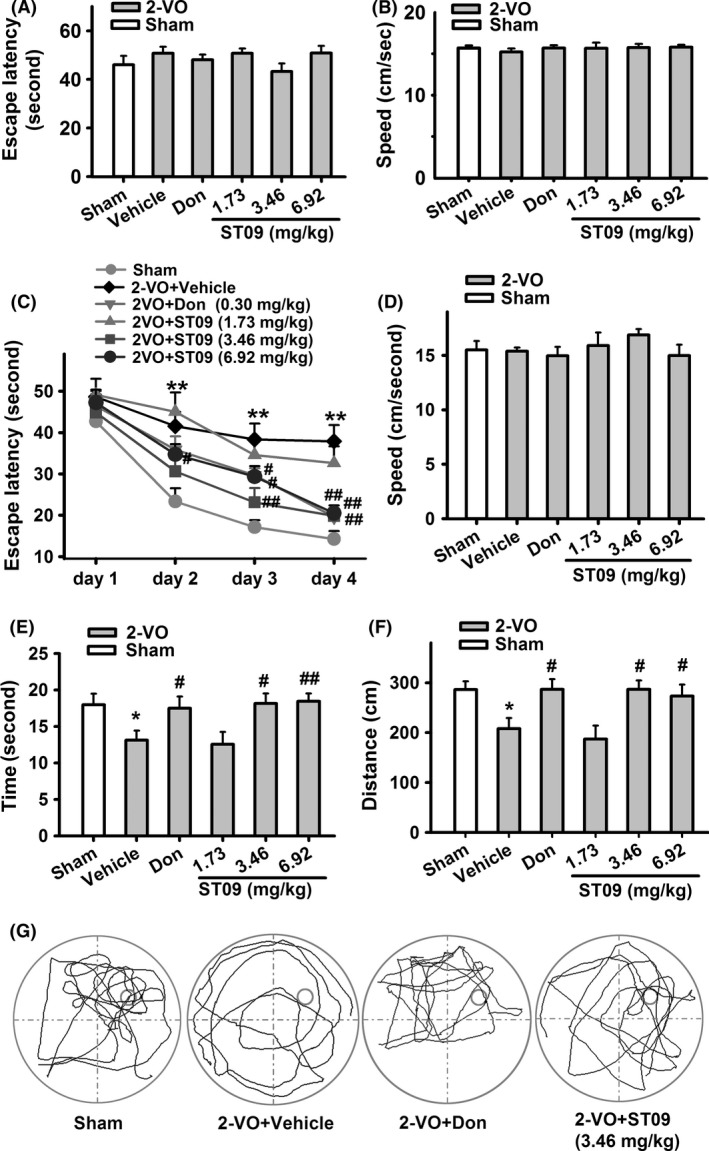
Effect of ST09 on the Morris water maze performance of rats. (A) Escape latency to find the visible platform. (B) Swimming speed in visible platform trials. There was no difference among ST09 (1.73 mg/kg: n = 8, 3.46 mg/kg: n = 9, 6.92 mg/kg: n = 9), Donepezil (Don, n = 9), sham (n = 9), and 2‐VO (n = 9) in escape latency (A) and speed (B), indicating that rats in all these groups have similar motor and visual capabilities. (C) Escape latency to find the hidden platform on days 1–4 (two‐way repeated‐measures ANOVA, followed by Turkey post hoc test). (D–F) Swimming speed (D) and time (E) and distance (F) in the target zone in the spatial probe test. (G) Swimming trace in the spatial probe test. The smaller cycle represents the platform, and the larger cycle represents the pool edge (ST09: 3.46 mg/kg). Data are presented as mean ± SEM. in all panels. *P < 0.05, **P < 0.01 vs. sham; # P < 0.05, ## P < 0.01 vs. 2‐VO by one‐way ANOVA followed by LSD multiple comparisons post hoc tests.
ST09 Inhibits the AChE Activity in the Hippocampus and Cortex of VaD Rats
We further investigated the mechanisms underlying its anti‐VaD effects. As shown in Table S4, both ST09 and its active metabolite ST10 produced potent ChE inhibitory effect. As shown in Figure 3A–B, compared with sham group, the activities of AChE were significantly increased in the hippocampus and cortex in the 2‐VO group (cortex: 107.38 ± 7.52%; hippocampus: 126.70 ± 6.01%, P < 0.05 vs. sham). Similar to donepezil, ST09 (3.46 mg/kg, 6 weeks) significantly reduced AChE activity by 22.86 ± 4.33% (P < 0.05 vs. 2‐VO) in the cortex and by 16.96 ± 4.90% (P < 0.01 vs. 2‐VO) in the hippocampus when compared with 2‐VO group. No significant changes in the BuChE activity were found among these groups (Figure 3B). These results indicate that inhibition of AChE may be involved in the anti‐VaD effect of ST09.
Figure 3.
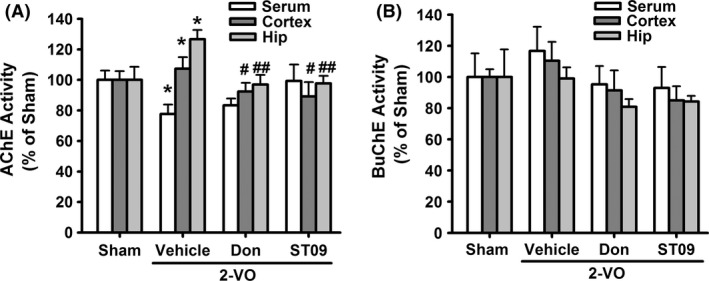
Inhibition of AChE and BuChE by mercapto‐tacrine derivatives in vivo. (A–B) The activities of AChE (A) and BuChE (B) in serum, cerebral cortex, and hippocampus of the rats. Data are presented as mean ± SEM. n = 5, *P < 0.05, **P < 0.01 vs. sham; # P < 0.05, ## P < 0.01 vs. 2‐VO by one‐way ANOVA followed by LSD multiple comparisons post hoc tests.
ST09 Reduces Oxidative Stress in the Hippocampus and Cortex of VaD Rats
In the ESR of N‐benzylidene‐tert‐butylamine N‐oxide (PBN) self‐spin (Figure 4Aa–e), both ST09 (100 μM and 200 μM) and ST10 (100 μM and 200 μM) significantly decreased the peak intensity of hydroxyl radical (80.29 ± 1.55% of vehicle group for 100 μM ST09, 71.66 ± 2.83% for 100 μM ST10, 65.36 ± 4.01% for 200 μM ST09, 42.78 ± 2.36% for 200 μM ST10, n = 6, P < 0.01 vs. vehicle), while tacrine at the same concentration did not exhibit this hydroxyl radical eliminating activity. Then, we investigated the antioxidation effect of ST09 in vivo. As shown in Figure 4B, when compared with sham group, 2‐VO induced a significant decrease in the level of protein‐bound sulfhydryl group in serum (78.38 ± 1.84%, P < 0.01, vs. sham), hippocampus (83.63 ± 1.60%, P < 0.01 vs. sham), and cortex (82.53 ± 2.19%, P < 0.01 vs. sham). ST09 (3.46 mg/kg, 6 weeks) significantly increased the level of protein‐bound sulfhydryl group in all these tissues (serum: 120.82 ± 3.88%; cortex: 121.20 ± 5.58%; hippocampus: 102.32 ± 2.12%; P < 0.01 vs. 2‐VO), which was much better than that of donepezil group. Compared with 2‐VO group, ST09 decreased the malondialdehyde (MDA) level by 45.30 ± 8.79% in the cortex and by 49.20 ± 6.34% in the hippocampus (P < 0.01, Figure 4C). Compared with sham group, superoxide dismutase (SOD) activity of 2‐VO group was decreased in the cortex (Figure 4D) from 450.07 ± 11.17 U/mg protein to 343.15 ± 18.59 U/mg, and in the hippocampus (Figure 4E) from 168.70 ± 5.51 U/mg to 151.83 ± 4.95 U/mg, respectively. These alterations were reversed by ST09, which was similar to the effect of donepezil (P < 0.01 or 0.05 vs. 2‐VO).
Figure 4.
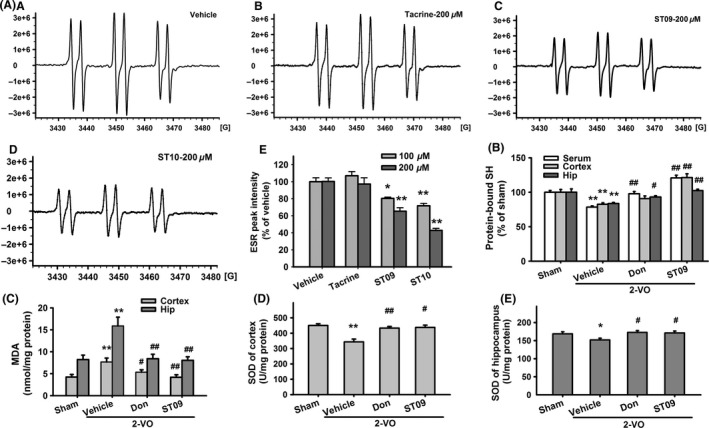
The effect of ST10 on free radical scavenging. (A) ESR spectra of HO radical detected in the reaction mixture containing 500 μM H2O2, 30 mM PBN, and 0.2 mM FeSO 4 (A) vehicle‐1% (v/v) DMSO, (b) 200 μM tacrine, (c) ST09, and (d) ST10. (e) Peak intensity of the HO ESR signal detected in the reaction mixture in the presence of vehicle, tacrine, ST09, and ST10. Data are presented as mean percentage of vehicle ± SEM. **P < 0.01 vs. vehicle. (B–E) Effects of ST09 on protein‐bound SH group level (B), MDA level (C), and SOD activity (D–E). Data are presented as mean ± SEM. n = 5–6. *P < 0.05, **P < 0.01 vs sham; # P < 0.05, ## P < 0.01 vs. 2‐VO by one‐way ANOVA followed by LSD multiple comparisons post hoc tests.
ST09 Ameliorates Neuronal Apoptosis and Pathological Damage in the Cortex and Hippocampus of VaD Rats
Neuronal apoptosis is a primary pathological mechanism in the development of VaD. Rats in the 2‐VO group showed a decrease in Bcl‐2/Bax ratio and an increase in the level of activated caspase‐3, compared with sham group. The Bcl‐2/Bax ratio in 2‐VO‐induced model was increased by ST09 (3.46 mg/kg, 6 weeks) from 40.08 ± 3.09% to 93.59 ± 5.82% (P < 0.05 vs. 2‐VO, n = 5) in the cortex, and from 26.43 ± 4.78% to 83.99 ± 5.87% (P < 0.01 vs. 2‐VO, n = 5) in the hippocampus (Figure 5A). ST09 (3.46 mg/kg, 6 weeks) also significantly inhibited the 2‐VO‐induced activation of caspase‐3 from 144.90 ± 15.38% to 97.45 ± 8.20% (P < 0.01 vs. 2‐VO, n = 8) in the cortex, and from 138.32 ± 8.30% to 85.24 ± 8.64% (P < 0.05 vs. 2‐VO, n = 5) in the hippocampus (Figure 5B). H&E staining was carried out to evaluate the morphology in the cortex and hippocampus. After chronic brain ischemia, rats in vehicle‐treated group showed remarkably shrinked neurons with pyknotic nucleus, indistinct nucleoli, and eosinophilic cytoplasm, and reduced cell density in both cerebral cortex and hippocampus CA1 area (Figure 5C). The sections selected at the same layer from each rat were assessed by counting of neurons (i.e., necrotic or apoptotic) at ×400, in 6 non‐overlapping fields in each ROI by an examiner blinded to the experimental protocol 29, 30. The number of necrotic neurons was divided by the total number of intact and necrotic neurons to derive a percentage. Necrotic neurons were classified by the presence of pyknosis, karyorrhexis, karyolysis, cytoplasmic eosinophilia (“red neuron”), or loss of affinity for hematoxylin (“ghost neuron”). The propotions of necrotic neurons in cerebral cortex and hippocampus CA1 area were 75 ± 8% and 42 ± 10%, respectively (Figure 5D), while no signfcant histological abnormalities were demonstrated in the sham group. Similar to donepezil, ST09 greatly attenuated chronic hypoperfusion‐induced morphologic changes in cerebral cortex and hippocampus. The propotions of necrotic neurons were decreased to 39 ± 26% and 16 ± 7% by ST09, which was significantly lower than that in vehicle‐treated group (P < 0.05 or 0.01). These results indicate that the anti‐VaD effect of ST09 is closely associated with its suppression of apoptosis.
Figure 5.
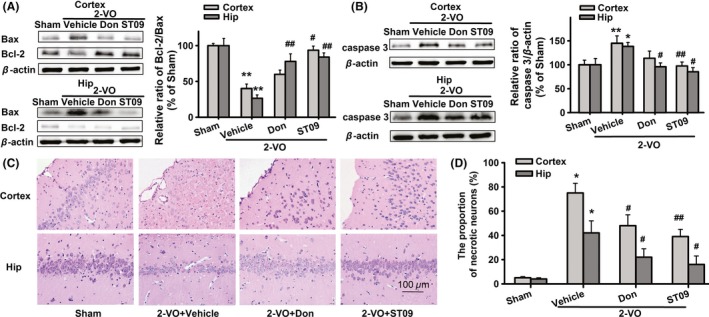
Effects of ST09 on the expression of Bax, Bcl‐2, and activated caspase‐3 proenzyme in the cortex and hippocampus of VaD rats. (A) Effects of ST09 (3.46 mg/kg) on the level of Bax and Bcl‐2 protein in VaD model. (B) Effects of ST09 (3.46 mg/kg) on protein level of activated caspase 3 in VaD rats. Values are means ± SEM. n = 5–8 (Bcl‐2/Bax: hip, n = 5; cortex, n = 5. caspase 3: hip, n = 5; cortex, n = 8) and normalized by β‐actin and expressed as folds. *P < 0.05, **P < 0.01 vs. sham; # P < 0.05, ## P < 0.01 vs. 2‐VO by one‐way ANOVA, followed by LSD multiple comparisons post hoc tests. (C) Representative photomicrographs of cortex and hippocampal CA1 area showing the histopathological changes of each group (H&E staining, × 400). (D) The proportion of necrotic neurons within the regions of cortex or hippocampus. Values are means ± SEM. n = 6. **P < 0.01 vs. sham, # P < 0.05, ## P < 0.01 vs. 2‐VO by one‐way ANOVA, followed by LSD multiple comparisons post hoc tests.
ST09 Enhances the Glucose Metabolism in the Cortex and Hippocampus of VaD Rats
Considering that deficits in energy metabolism play a central role in the pathophysiology of VaD 31, we further investigated the effect of ST09 on glucose metabolism in 2‐VO‐induced VaD model using the regional cerebral metabolic rate for glucose by the 18F‐FDG‐PET. The volumes of interest (VOI) reflect the declines in glucose metabolism. In these images, different color indicates different 18F‐FDG uptake levels. We found that 2‐VO induced an obvious inhibition on 18F‐FDG uptake from 1.33 ± 0.03% ID/g to 0.77 ± 0.08% ID/g in the cortex (P < 0.01 vs. sham, n = 5), and from 1.15 ± 0.06% ID/g to 0.87 ± 0.05% ID/g in the hippocampus (P < 0.01 vs. sham, n = 5). Compared with 2‐VO group, FDG accumulation significantly increased to 1.13 ± 0.07% ID/g (P < 0.01, vs. 2‐VO) in the cortex, and 1.11 ± 0.05% ID/g (P < 0.05 vs. 2‐VO) in the hippocampus after ST09 treatment (Figure 6A–B). However, donepezil showed little effect on the deficit of glucose metabolism in these two brain regions of VaD rats (cortex: 0.91 ± 0.07% ID/g; hippocampus: 0.94 ± 0.07% ID/g, P > 0.05 vs. 2‐VO), suggesting that improved glucose utilization could be responsible for the novel potential therapeutic effect of ST09.
Figure 6.
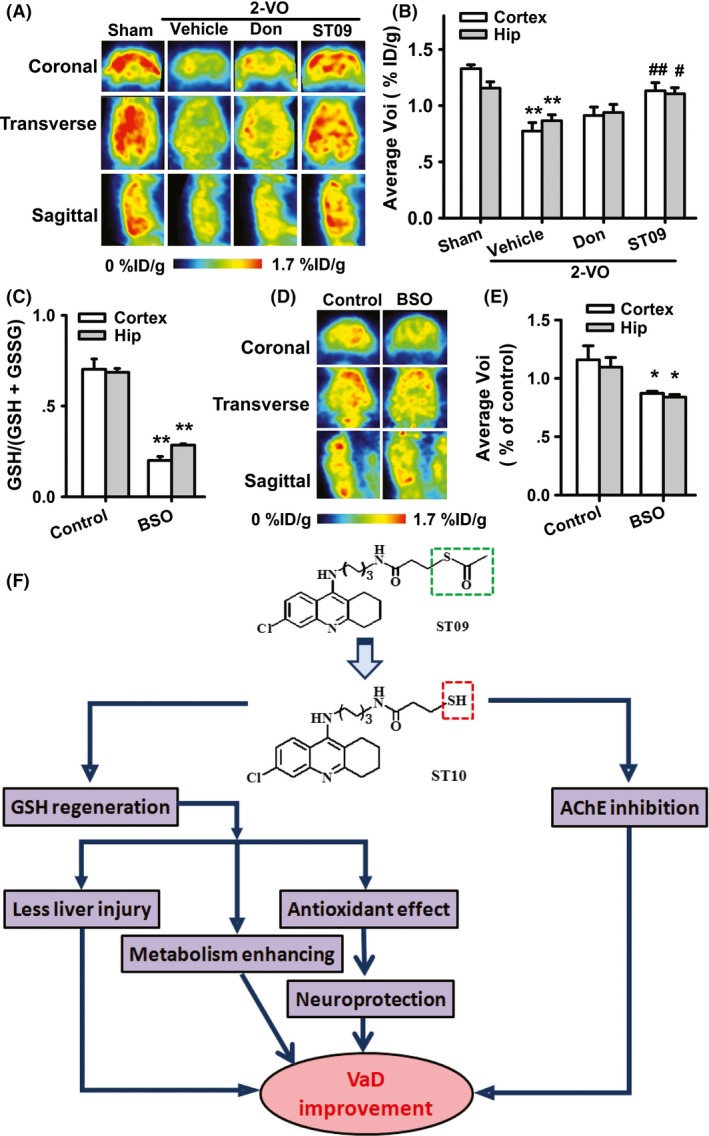
Images of 18 FDG‐PET. (A–B) Effect of ST09 on the glucose metabolism in VaD rats. Values are means ± SEM. n = 5. **P < 0.01 vs. sham, # P < 0.05, ## P < 0.01 vs. 2‐VO by one‐way ANOVA, followed by LSD multiple comparisons post hoc tests. (C) The GSH depletion by intra‐cerebroventricular injection BSO. (D–E) Effect of BSO on glucose metabolism in rats. Values are means of control ± SEM. n = 5. *P < 0.05, **P < 0.01 vs. control by one‐way ANOVA, followed by LSD multiple comparisons post hoc tests. (F) Pathways of ischemic vascular dementia and possible multifunctional therapeutic mechanisms of ST09.
To confirm the role of ST09‐induced GSH regeneration in its effects on metabolism, we observed the energy metabolism in the cortex and hippocampus of rats with GSH depletion. Intra‐cerebroventricular injection of L‐buthionine sulfoximine (L‐BSO, 3.20 mg/rat, 8 h, n = 5) significantly decreased the GSH levels in both cortex and hippocampus of rats (P < 0.01 vs. control, shown in Figure 6C). PET scanning graphs showed that the rate of glucose metabolism in the brain of BSO‐treated rats was downregulated from 1.16 ± 0.12% ID/g to 0.87 ± 0.02 ID/g in the cortex and from 1.10 ± 0.08% ID/g to 0.84 ± 0.02% ID/g in hippocampus (Figure 6D–E, P < 0.05 vs. control, n = 5), indicating that thiol redox status affects glucose metabolism.
Discussion
In the present study, we found that ST09, a thioester derivative of tacrine, significantly ameliorated chronic cerebral hypoperfusion‐induced cognitive impairment in rats. This effect derived from multiple mechanisms, including AChE inhibition, free radical scavenging capacity, neuroprotection, and metabolism improvement, as shown in Figure 6F.
AChEIs such as donepezil are widely used in clinical trials of VaD 28, 29. In the MWM test, we found that the thioester ST09 improved the cognitive deficits in VaD rats, which was similar to donepezil. ST09 also significantly reduced AChE activity in the cortex and hippocampus. The anti‐VaD effect of ST09 may be largely dependent on their AChE inhibition. Interestingly, a series of AChEIs exhibit neuroprotection 33. In our present study, both donepezil and ST09 attenuated the neuronal apoptosis and ameliorated the pathological damage in VaD rats. Furthermore, we found that two more pharmacological properties were generated by the introduction of a mercapto group. One is the ability to scavenge free radicals; the other is the improvement of the energy metabolism. Under pathological conditions, oxidative stress triggers a decrease in the levels of GSH. Previous reports have indicated that thiol depletion may not only decrease the cellular antioxidant defense, but also alter the cellular metabolism status via two distinct mechanisms. One mechanism is the redox control of the mitochondria function, which is dependent on mitochondrial density and glutathione availability. Various mitochondrial proteins such as Complex I and ATP synthase are regulated by cysteine oxidation reactions, followed by inhibition of ATP synthesis and electron transport chain 34, 35, 36. Emerging evidence has shed a light on mitochondrial cysteine oxidation to mitochondrial dysfunction. The other mechanism is the redox control of glucose transporter (GLUT) activity. When the reductive activity of key cysteine residues is elevated in GLUT1, such as Cys351 and Cys361, the glucose transport will be activated and facilitated acutely 37, 38. This activity would be inhibited under oxidative stress. In our study, the use of animal PET scan made it possible to find the evidence that GSH depletion may contribute to 2‐VO‐induced deficits in energy metabolism in the brain. It is intriguing that depletion of the brain GSH level in vivo by intra‐cerebroventricular injection of L‐BSO significantly downregulated the glucose metabolism rate in the cortex and hippocampus. Together with previous studies, our current results further revealed a closely link between redox status and brain energy metabolism. Interestingly, pyritinol, a cerebral metabolic enhancer that improves brain metabolism in patients, also contains a potential thiol group and might release free thiol in vivo 39. Here, we found that ST09 exhibited a special improvement of brain metabolism in the VaD rats, which may be derived from the thiol regeneration. Further investigation is required to identify the precise target for mercaptan‐mediated improvement of brain metabolism.
The main limitation for tacrine is its serious hepatotoxicity. Our work confirmed previous findings that the hepatotoxicity of tacrine was attributed to tacrine‐induced ROS production 7, 18. It is noteworthy that the side effect of tacrine was reduced by the introduction of a potential mercapto group. Thus, ST09 exhibited the superior drug‐like properties. Although the ratio of GPT/GOT was not altered by ST09 administration for 6 weeks, long‐term evaluation may be required. Besides hepatotoxicity, the peripheral side effect of tacrine was attributed to its non‐specific action on BuChE, a cholinesterase widely expressed in peripheral tissues 40. Compared with tacrine, ST09 showed a higher selectivity to AChE than to BuChE, which may lead to less peripheral side effects.
In recent years, an ever‐increasing number of efforts to discover multitarget drugs have appeared in the neurodegenerative diseases 41, 42, 43. Here, ST09 is multifunctional with at least four types of mechanisms (Figure 6F): Firstly, it retains the classic anticholinesterase activity. In clinics, AChEIs are the first‐line agents for treatment of VaD 32, and the powerful cholinergic effect of ST09 is an important basis for its therapeutic effect. Secondly, the activity to scavenge free radical, which is inherently associated with thiol group, will produce a better neuroprotective effect. Thirdly, like pyritinol, ST09 releases free thiol group in vivo and promotes cerebral metabolism, which will be of great value in clinical application. Finally, according to our previous studies, mercaptan and mercapto‐tacrines enhances the hippocampal LTP 17, which may bring a direct cognitive‐enhancing effect. Most of these features are directly related to the potential thiol group, suggesting that the mercaptan‐containing ST09 may be a promising agent for the treatment of VaD.
Taken together, our study revealed that ST09 exhibited a therapeutic potential for VaD and introducing a potential mercapto group into the parent structure of classic AChEIs, such as tacrine, may serve as a promising strategy for the development of novel agents for VaD therapy.
Conflict of Interest
The authors declare no conflict of interests.
Supporting information
Figure S1. The effect of ST09 on the cultured HepG2 cells and the liver of VaD rat. (A, B) Effects of different concentrations of tacrine (A) and ST09 (0.1, 1, 10,100 μM) (B) on cell viability in HepG2 cells after 12 h treatment. (C, D) The effect of tacrine (C) and ST09 (D) on the intracellular production of ROS. (E, F) The effect of tacrine (E) and ST09 (F) on GSH level. Data are presented as mean percentage of control ± SEM from five independent experiments. *P < 0.05, **P < 0.01 versus vehicle, one‐way ANOVA followed by LSD multiple comparisons post hoc test. (G) Influence of ST09 on the activities of GPT (U/L) and GOT (U/L), n = 8. There were no significant difference between these four groups. (H) Representative photomicrographs of liver tissues showing no remarkable histopathological changes except tacrine group (H&E staining, ×400, tacrine: 3 mg/kg/day, ip, 6 week; n = 6).
Table S1. The peak area value of ST09 and ST10 at different time points after incubation in rat blood or saline in vitro.
Table S2. The peak area value of ST09 and ST10 at different time points in vivo.
Table S3. Blood concentration‐time profile of ST10 (i.p. 5 mg/kg). Values are means ± SEM. n = 6.
Table S4. Inhibition of AChE and BuChE (pIC50 values) by mercapoto‐tacrine derivatives in vitro.
Acknowledgment
This work was supported by grants from the National Science and Technology Major Projects for “New Drugs Innovation and Development” (2012ZX09103‐101‐045), the National Basic Research Program of China (973 Program, No. 2013CB531303), the International Science & Technology Cooperation Program of China (No. 2011DFA32670), the National Major Scientific Instrument and Equipment Development Projects (2013YQ03092306), and PCSIRT (No. IRT13016) to J.G.C.
The first two authors contributed equally to this work.
References
- 1. Gorelick PB, Scuteri A, Black SE, et al. Vascular contributions to cognitive impairment and dementia: A statement for healthcare professionals from the American heart association/American stroke association. Stroke 2011;42:2672–2713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Konno S, Meyer JS, Terayama Y, Margishvili GM, Mortel KF. Classification, diagnosis and treatment of vascular dementia. Drugs Aging 1997;11:361–373. [DOI] [PubMed] [Google Scholar]
- 3. Grantham C, Geerts H. The rationale behind cholinergic drug treatment for dementia related to cerebrovascular disease. J Neurol Sci 2002;203–204:131–136. [DOI] [PubMed] [Google Scholar]
- 4. Roman GC, Kalaria RN. Vascular determinants of cholinergic deficits in Alzheimer disease and vascular dementia. Neurobiol Aging 2006;27:1769–1785. [DOI] [PubMed] [Google Scholar]
- 5. Ames DJ, Bhathal PS, Davies BM, Fraser JR. Hepatotoxicity of tetrahydroaminoacridine. Lancet 1988;1:887. [DOI] [PubMed] [Google Scholar]
- 6. Fang L, Appenroth D, Decker M, et al. NO‐donating tacrine hybrid compounds improve scopolamine‐induced cognition impairment and show less hepatotoxicity. J Med Chem 2008;51:7666–7669. [DOI] [PubMed] [Google Scholar]
- 7. Osseni RA, Debbasch C, Christen MO, Rat P, Warnet JM. Tacrine‐induced reactive oxygen species in a human liver cell line: The role of anethole dithiolethione as a scavenger. Toxicol In Vitro 1999;13:683–688. [DOI] [PubMed] [Google Scholar]
- 8. Yue J, Dong G, He C, et al. Protective effects of thiopronin against isoniazid‐induced hepatotoxicity in rats. Toxicology 2009;264:185–191. [DOI] [PubMed] [Google Scholar]
- 9. Lukas RJ, Morimoto H, Bennett EL. Effects of thio‐group modification and Ca2 + on agonist‐specific state transitions of a central nicotinic acetylcholine receptor. Biochemistry 1979;18:2384–2395. [DOI] [PubMed] [Google Scholar]
- 10. Robillard JM, Gordon GR, Choi HB, Christie BR, MacVicar BA. Glutathione restores the mechanism of synaptic plasticity in aged mice to that of the adult. PLoS ONE 2011;6:e20676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Izigov N, Farzam N, Savion N. S‐allylmercapto‐N‐acetylcysteine up‐regulates cellular glutathione and protects vascular endothelial cells from oxidative stress. Free Radic Biol Med 2011;50:1131–1139. [DOI] [PubMed] [Google Scholar]
- 12. Stoica E, Meyer JS, Kawamura Y, et al. Central neurogenic control of cerebral circulation. Effects of intravertebral injection of pyrithioxin on cerebral blood flow and metabolism. Neurology 1973;23:687–698. [DOI] [PubMed] [Google Scholar]
- 13. Arakawa M, Ito Y. N‐acetylcysteine and neurodegenerative diseases: Basic and clinical pharmacology. Cerebellum 2007;6:308–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Pocernich CB, Butterfield DA. Elevation of glutathione as a therapeutic strategy in Alzheimer disease. Biochim Biophys Acta 2012;1822:625–630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Deneke SM. Thiol‐based antioxidants. Curr Top Cell Regul 2000;36:151–180. [DOI] [PubMed] [Google Scholar]
- 16. Yang YJ, Wu PF, Long LH, et al. Reversal of aging‐associated hippocampal synaptic plasticity deficits by reductants via regulation of thiol redox and NMDA receptor function. Aging Cell 2010;9:709–721. [DOI] [PubMed] [Google Scholar]
- 17. Cai F, Wang F, Lin FK, et al. Redox modulation of long‐term potentiation in the hippocampus via regulation of the glycogen synthase kinase‐3beta pathway. Free Radic Biol Med 2008;45:964–970. [DOI] [PubMed] [Google Scholar]
- 18. Minarini A, Milelli A, Simoni E, et al. Multifunctional tacrine derivatives in Alzheimer's disease. Curr Top Med Chem 2013;13:1771–1786. [DOI] [PubMed] [Google Scholar]
- 19. Wang Y, Guan XL, Wu PF, et al. Multifunctional mercapto‐tacrine derivatives for treatment of age‐related neurodegenerative diseases. J Med Chem 2012;55:3588–3592. [DOI] [PubMed] [Google Scholar]
- 20. Wang H, Joseph JA. Quantifying cellular oxidative stress by dichlorofluorescein assay using microplate reader. Free Radic Biol Med 1999;27:612–616. [DOI] [PubMed] [Google Scholar]
- 21. Padilla S, Lassiter TL, Hunter D. Biochemical measurement of cholinesterase activity. Methods Mol Med 1999;22:237–245. [DOI] [PubMed] [Google Scholar]
- 22. Sedlak J, Lindsay RH. Estimation of total, protein‐bound, and nonprotein sulfhydryl groups in tissue with Ellman's reagent. Anal Biochem 1968;25:192–205. [DOI] [PubMed] [Google Scholar]
- 23. Borthiry GR, Antholine WE, Kalyanaraman B, Myers JM, Myers CR. Reduction of hexavalent chromium by human cytochrome b5: Generation of hydroxyl radical and superoxide. Free Radic Biol Med 2007;42:738–755, 735‐737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Fan H, Wu PF, Zhang L, et al. Methionine sulfoxide reductase A negatively controls microglia‐mediated neuroinflammation via inhibiting ROS/MAPKs/NF‐kappaB signaling pathways through a catalytic antioxidant function. Antioxid Redox Signal 2015;22:832–847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Liu C, Wu J, Gu J, et al. Baicalein improves cognitive deficits induced by chronic cerebral hypoperfusion in rats. Pharmacol Biochem Behav 2007;86:423–430. [DOI] [PubMed] [Google Scholar]
- 26. Qingguo X, Yuanbao C, Jun Z, et al. Implementation of LYSO/PSPMT Block Detector With All Digital DAQ System. Nucl Sci IEEE Trans On 2013;60:1487–1494. [Google Scholar]
- 27. Qingguo X, Luyao W, Jun Z, et al. Development and initial performance measurements of Trans‐PET BioCaliburn SH1.0. In, Nuclear Science Symposium And Medical Imaging Conference (Nss/Mic), 2012 Ieee; 2012:3090‐3092.
- 28. Erkinjuntti T, Roman G, Gauthier S, Feldman H, Rockwood K. Emerging therapies for vascular dementia and vascular cognitive impairment. Stroke 2004;35:1010–1017. [DOI] [PubMed] [Google Scholar]
- 29. Wang J, Zhang HY, Tang XC. Cholinergic deficiency involved in vascular dementia: Possible mechanism and strategy of treatment. Acta Pharmacol Sin 2009;30:879–888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Fowler JS, Ido T. Initial and subsequent approach for the synthesis of 18FDG. Semin Nucl Med 2002;32:6–12. [DOI] [PubMed] [Google Scholar]
- 31. Sicard KM, Henninger N, Fisher M, et al. Long‐term changes of functional MRI–based brain function, behavioral status, and histopathology after transient focal cerebral ischemia in rats. Stroke 2006;37:2593–2600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Li Y, Powers C, Jiang N, et al. Intact, injured, necrotic and apoptotic cells after focal cerebral ischemia in the rat. J Neurol Sci 1998;156:119–132. [DOI] [PubMed] [Google Scholar]
- 33. Sugimoto H. Scope and limitations of acetylcholinesterase inhibitors. Nihon Yakurigaku Zasshi 2004;124:163–170. [DOI] [PubMed] [Google Scholar]
- 34. Mailloux RJ, Jin X, Willmore WG. Redox regulation of mitochondrial function with emphasis on cysteine oxidation reactions. Redox Biol 2014;2:123–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Wang SB, Murray CI, Chung HS, Van Eyk JE. Redox regulation of mitochondrial ATP synthase. Trends Cardiovasc Med 2013;23:14–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Louters LL, Scripture JP, Kuipers DP, et al. Hydroxylamine acutely activates glucose uptake in L929 fibroblast cells. Biochimie 2013;95:787–792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Burgman P, Odonoghue JA, Humm JL, Ling CC. Hypoxia‐Induced increase in FDG uptake in MCF7 cells. J Nucl Med 2001;42:170–175. [PubMed] [Google Scholar]
- 38. Xia X, Wang G, Peng Y, Jen J. Cys351 and Cys361 of the Na+/glucose cotransporter are important for both function and cell‐surface expression. Arch Biochem Biophys 2005;438:63–69. [DOI] [PubMed] [Google Scholar]
- 39. Martin KJ, Tucker L, Widdowson L. Effects of some metabolites of pyritinol (Encephabol) on ACH release from brain slices. Pharmacopsychiatry 1988;21(Suppl 1):33–36. [DOI] [PubMed] [Google Scholar]
- 40. Pacheco G, Palacios‐Esquivel R, Moss DE. Cholinesterase inhibitors proposed for treating dementia in Alzheimer's disease: Selectivity toward human brain acetylcholinesterase compared with butyrylcholinesterase. J Pharmacol Exp Ther 1995;274:767–770. [PubMed] [Google Scholar]
- 41. Anighoro A, Bajorath J, Rastelli G. Polypharmacology: challenges and opportunities in drug discovery. J Med Chem 2014;57:7874–7887. [DOI] [PubMed] [Google Scholar]
- 42. Leon R, Marco‐Contelles J. A step further towards multitarget drugs for Alzheimer and neuronal vascular diseases: Targeting the cholinergic system, amyloid‐beta aggregation and Ca(2 + ) dyshomeostasis. Curr Med Chem 2011;18:552–576. [DOI] [PubMed] [Google Scholar]
- 43. Liu X, Zhu F, Ma XH, et al. Predicting targeted polypharmacology for drug repositioning and multi‐ target drug discovery. Curr Med Chem 2013;20:1646–1661. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. The effect of ST09 on the cultured HepG2 cells and the liver of VaD rat. (A, B) Effects of different concentrations of tacrine (A) and ST09 (0.1, 1, 10,100 μM) (B) on cell viability in HepG2 cells after 12 h treatment. (C, D) The effect of tacrine (C) and ST09 (D) on the intracellular production of ROS. (E, F) The effect of tacrine (E) and ST09 (F) on GSH level. Data are presented as mean percentage of control ± SEM from five independent experiments. *P < 0.05, **P < 0.01 versus vehicle, one‐way ANOVA followed by LSD multiple comparisons post hoc test. (G) Influence of ST09 on the activities of GPT (U/L) and GOT (U/L), n = 8. There were no significant difference between these four groups. (H) Representative photomicrographs of liver tissues showing no remarkable histopathological changes except tacrine group (H&E staining, ×400, tacrine: 3 mg/kg/day, ip, 6 week; n = 6).
Table S1. The peak area value of ST09 and ST10 at different time points after incubation in rat blood or saline in vitro.
Table S2. The peak area value of ST09 and ST10 at different time points in vivo.
Table S3. Blood concentration‐time profile of ST10 (i.p. 5 mg/kg). Values are means ± SEM. n = 6.
Table S4. Inhibition of AChE and BuChE (pIC50 values) by mercapoto‐tacrine derivatives in vitro.


