Summary
Aims
Individuals with Smith–Magenis syndrome (SMS) are reported to have a disrupted circadian rhythm. Our aim was to examine problematic sleeping in those attending our sleep clinic for the first time.
Methods
At intake, caregivers of 50 children and nine adults with SMS were surveyed about the sleep pattern and potential melatonin administration. Sampling of salivary melatonin levels was performed.
Results
At intake, exogenous melatonin was used by 16 children (27.1% of sample; 56.3% male) with mean age 6.8 ± 2.8 years, whereas 34 children (57.6%; 7.5 ± 4.8 years old; 64.7% male) and nine adults (15.3%; 36.8 ± 15.3 years old; 44.4% male) were not taking melatonin at intake. Participants were reported to have problems with night waking and early awakenings regardless of melatonin administration. Overall, moderate to high levels of salivary melatonin at noon were found in individuals with SMS. In particular, children with SMS showed a disrupted melatonin pattern. Furthermore, the endogenous melatonin level, age, and gender may potentially interact, yielding the severity range of sleep disturbances reported in SMS.
Conclusion
Treatment of sleep problems in SMS is complex, and our findings may support person‐centered sleep and medication management. Future clinical trials including larger groups may shed light on such approaches.
Keywords: Awakening, Circadian rhythm, Insomnia, Melatonin, Sleep, Sleep onset, Smith–Magenis syndrome
Introduction
Melatonin, which is an exogenously administered food supplement regulating the body clock, is frequently given to treat sleep problems in individuals with developmental disabilities 1, 2, 3, 4. Melatonin should be administered at a time related to dim light melatonin onset, which refers to the start of the endogenous melatonin production in the evening during dim light conditions 4. However, potential concerns regarding quality, reliability, and unpredictable pharmacokinetic properties of melatonin preparations (e.g., the type of melatonin), and hence safety, remain 5. The efficacy of exogenous melatonin is therefore increasingly studied in individuals with developmental disabilities (e.g., 2, 3, 6). Although sleep complaints and their management in individuals with developmental disabilities are commonly reported in literature, more rigorous studies (e.g., clinical trials, large samples) delineating problematic sleeping and its management in individuals with developmental disabilities are needed 7.
Smith–Magenis syndrome (SMS; OMIM 182290) is an intellectual disability syndrome with distinctive behavioral characteristics (e.g., tantrums), dysmorphic features, and congenital anomalies (e.g., heart and kidney defects) ascribed to an interstitial deletion of chromosome 17p11.2 8 or RAI1 mutation 9. Disrupted sleep patterns or parent‐reported challenging nighttime behavior is highly characteristic 10, 11. Individuals with SMS are reported to have an early sleep onset (i.e., between 19:30 and 20:30), repeated and prolonged waking at night, and an early sleep offset (04:00–05:00) 12, 13. Night waking tends to last long 14, but primarily the individuals are described as hyperactive 13, 14, 15. Their hyperactivity at night forces caregivers to undertake actions such as lock doors, switch lights. Individuals with SMS are further experiencing daytime somnolence and a decreased total sleep time 16. Characteristic to SMS is a potentially abnormal or a shifted circadian rhythm 12, 17. However, to date, it remains unclear whether their disrupted sleep rhythm might be ascribed to for instance insufficient melatonin production, deranged circadian secretion profile, melatonin receptor insensitivity.
Endogenous melatonin is released by the pineal gland during darkness and is a marker of our circadian phase 18. In typically developing individuals, melatonin levels are the lowest during daytime and highest during nighttime. Moreover, the amplitude of the melatonin level varies among typically developing individuals. But also across their life span, levels of melatonin may vary, that is, an age‐related decline has been found with a turning point being puberty 19. Gender differences in melatonin levels of typically developing individuals have likewise been suggested. Girls were found to secrete more melatonin 19, 20. On the contrary, in the majority of individuals with SMS, increased daytime levels of salivary melatonin were found 21. Previous studies have linked the severe sleep disturbances and maladaptive daytime behaviors characteristic of individuals with SMS to this abnormal circadian secretion pattern of melatonin 12, 13, 14, 17, 22, 23. In addition, an early onset and offset of melatonin secretion with a peak at noon in urine and plasma was found following hourly assessments 12. A protracted melatonin secretion of about 15 h, without alternations in other biological rhythms such as cortisol or growth hormone secretions, was therefore hypothesized by De Leersnyder et al. 12. More recently, Boone et al. 22 questioned a true inversion and speculated on a phase advance or delay of melatonin secretion of about half a day in SMS. In spite of these findings, the mechanism of the inversed or shifted melatonin secretion pattern in SMS is unknown. Similarly, the age‐related or gender differences in levels of melatonin found in typically developing individuals have been infrequently investigated in samples of individuals with SMS.
Given that each of the participants or their caregiver attended our sleep clinic with a sleep complaint, our objective was assessment for effective treatment. Our aims were 3‐fold: (1) to assess who was taking melatonin; (2) to evaluate the 24‐h level of melatonin in individuals with SMS with and without melatonin use at intake; and (3) to compare caregiver‐reported Sleep Onset Latency (SOL), number of Night Waking (NW) and Early Awakening (EW) complaints of individuals with SMS with and without melatonin use at intake.
Materials and Methods
Participants
This study was approved by the Institutional Review Board of Maastricht University. We obtained informed consent from parents and when applicable assent from children. Participants were recruited from Governor Kremers sleep clinic at ‘s Heeren Loo, the Netherlands.
Procedure
At intake, caregivers of individuals with SMS were asked about their melatonin use and their sleep pattern. We poststratified the sample on age, gender, and melatonin use at intake to evaluate their sleep in realm of the age‐related or gender differences in levels of melatonin found in typically developing participants. Assessment of melatonin will be reported at intake, at the start of the treatment in our clinic, and when caregivers of individuals with SMS reported that the initial sleep problems were no longer present (e.g., fewer awakenings; i.e., effective dose of melatonin).
Measures
Melatonin
At first, consultative participants with sleep problems attending our clinic are customarily asked to assess the 24‐h level of melatonin. It is requested to collect saliva hourly from 12 PM till noon next day. Salivary assessment 21 at first consult is primarily carried out to ascertain whether melatonin levels are high. Saliva was collected in Salivette® tubes (Sarstedt, Nümbrecht, Germany) by chewing on a cotton swab for 1–2 min. Melatonin levels were measured in saliva with a Radio Immuno Assay (RIA) (Buhlmann laboratories, Schönenbuch, Switzerland) as previously described 24. Radio activity was counted with the Perkin Elmer 1470 Wizard gamma counter (PerkinElmer Nederland B.V., Groningen, the Netherlands). The linear range is between 0.5 and 50 pg/mL. Salivary melatonin was categorized as low (≤0.5 pg/mL), moderate (0.5–50 pg/mL), and high (≥50 pg/mL).
Sleep Pattern
Caregivers provided the best estimate of Bedtime (BT), duration of Sleep Onset Latency (SOL), the number of Night Waking (#NW) and duration of Night Waking (NW), and the time of Wake Up (WU).
Statistical Analysis
Six groups will be described given the poststratification of the total sample on age, gender, and melatonin use at intake, that is, girls (12%, n = 7) and boys (15%, n = 9) using exogenous melatonin at intake, girls (20%, n = 12) and boys (37%, n = 22) not using exogenous melatonin at intake, women (9%, n = 5) and men (7%, n = 4) not using exogenous melatonin at intake of the total sample. Statistical descriptions were made using the mean (M) and standard deviation (SD), and percentage for categorical variables.
Results reflect a convenience sample in which the serial sampling or clinical data collection by the caregiver was not continuously performed or there was insufficient saliva collected. Analysis of missing data showed that missing data were random. Melatonin results will be presented as a proportion of the total sample including missing data. This allows comparison of groups, without findings being affected by the missing data and reflecting the same sample of individuals with SMS.
Bedtime, SOL, NW, and WU were separately compared through the nonparametric Kruskal–Wallis one‐way analysis of variance because of small sample sizes. Regarding the sleep pattern, also the percentage variation in mean within each group was calculated. This allows immediate comparison between data with different units.
When possible, a secondary analysis per genotype will be pursued. Two‐tailed P‐values will be printed. Statistical analyses were performed with Statistica version 10 (StatSoft, Inc. (2009), STATISTICA, Tulsa, OK, USA).
Results
Caregivers of 50 children (<18 years) and nine adults agreed to participate. SMS was diagnosed on genetic deletion in 78%, genetic mutation in 18%, and clinical features in 4% of the total sample. All participants have sleep problems for which they attended the sleep clinic. At intake, exogenous melatonin was used by 16 children (27% of sample; 56% male) with mean age of 7 years (SD = 3). There was no significant relationship between age and exogenous melatonin doses at intake (Spearman correlation: 0.4, P = 0.11; Figure 1). However, 34 children (58%; 8 ± 5 years old; 65% male) and nine adults (15%; 37 ± 15 years old; 44% male) were not taking melatonin at intake (of note, the sample did not include adults on melatonin at intake). On average, 4.5 ± 2.7 mg (min: 0.3–max: 10 mg) of melatonin was administered to those (n = 16, 27% of the sample) using melatonin at intake. Clinical data collection by the caregiver was mostly performed at 12 PM, 3 PM, 6 PM, 9 PM, 9 AM, and 12 PM next day.
Figure 1.
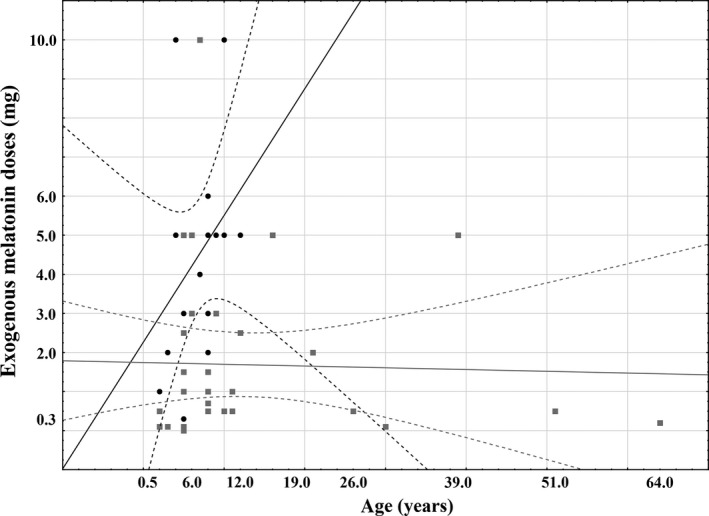
Scatter plot of exogenous melatonin doses by age. Solid Line: linear relationship; doted Line: 95% Confidence Interval; black dots: melatonin doses at intake; gray squares: effective melatonin doses.
Step 1: The 24‐h Response Curve at Intake
Total Group
The levels of salivary melatonin (see Figure 2) were proportionally different between the six groups at noon. More specifically, at the first assessment (χ2 (5) = 17.5, P = 0.004), more boys on exogenous melatonin have a high level, fewer of them have a moderate level, and without prior melatonin use, fewer boys have a high level in comparison with others. At 2 PM (χ2 (4) = 13.0, P = 0.03), proportionally more children, that is boys and girls, on exogenous melatonin have a high level. At 4 PM, more boys on exogenous melatonin at intake (χ2 (4) = 12.1, P = 0.01) have a high level and fewer have a moderate level. No data in boys on exogenous melatonin at intake were collected, but at 5 PM, more girls on exogenous melatonin at intake (χ2 (2) = 9.0, P = 0.03) have a high level. In the early morning (6 AM), albeit statistically borderline, more boys and men not on exogenous melatonin at intake (χ2 (10) = 16.3, P = 0.05) have a low level.
Figure 2.
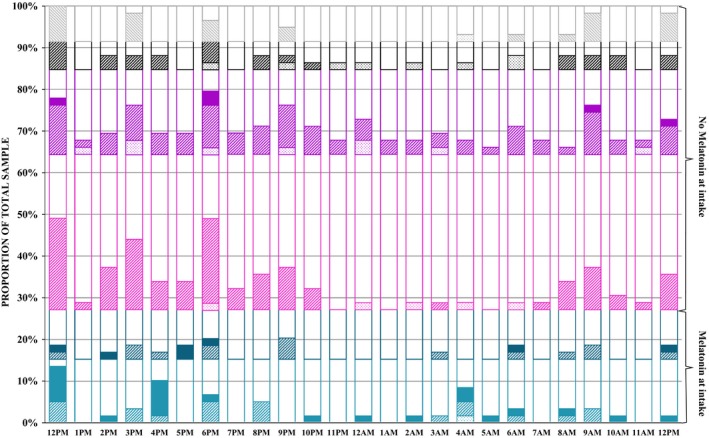
Proportional distribution of low, moderate, and high salivary melatonin levels of the total sample (six groups) at intake. Salivary levels of melatonin: Dotted fill: <0.50pg/ml; striped fill: between 0.5–50pg/ml; complete fill: >50pg/ml; no fill: missing. Groups: Children ‐ Light cyan: boys taking melatonin at intake; dark cyan: girls taking melatonin at intake; light magenta: boys not taking melatonin at intake; dark magenta: girls not taking melatonin at intake; adults ‐ black: men not taking melatonin at intake and grey: women not taking melatonin at intake.
Genetic Deletion (GD) group
The total sample comprised 43 participants with a genetic deletion, that is, of the GD sample, girls (14%, n = 6) and boys (14%, n = 6) using exogenous melatonin at intake, girls (21%, n = 9) and boys (40%, n = 17) not using exogenous melatonin at intake, women (7%, n = 3) and men (5%, n = 2) not using exogenous melatonin at intake. At intake, significantly more boys and men on exogenous melatonin have high melatonin levels in comparison with others (χ2 (4) = 16.0, P = 0.003). Yet, no differences in melatonin levels across 24 h were found across the six groups within participants with a genetic deletion.
Genetic Mutation (GM) group
Ten participants with genetic mutation participated, that is, of the GM sample, girls (20%, n = 2) and boys (10%, n = 1) using exogenous melatonin at intake, girls (40%, n = 4) and boys (10%, n = 1) not using exogenous melatonin at intake, women (20%, n = 2) not using exogenous melatonin at intake. No differences in melatonin levels were found across the five groups within participants with a genetic mutation.
Clinical features group was too small to perform separate analyses.
Sleep Complaints at Intake
The six groups were similar regarding sleep complaints (see Table 1). However, substantial variation in means potentially associated with an interaction effect was noted, that is, girls and women without prior melatonin use exhibited the highest variation in SOL. With regard to night waking, the boys without prior melatonin usage showed the largest variation. A large variation in WU was primarily observed in girls on melatonin.
Table 1.
Descriptive statistics of parent‐reported sleep complaints
| Melatonin at intake | No melatonin at intake | Kruskal–Wallis test | P‐value | |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Boys | Girls | Boys | Girls | Men | Women | |||||||||||||||
| M | SD | V | M | SD | V | M | SD | V | M | SD | V | M | SD | V | M | SD | V | |||
| BT (h) | 19.2 | 0.8 | 4.0 | – | – | – | 19.5 | 0.6 | 3.1 | 20.1 | 0.9 | 4.4 | – | – | – | 19.0 | – | – | H(5, N = 11) = 0.00 | 1.00 |
| SOL (min) | 5.7 | 1.9 | 33.1 | 8.3 | 8.2 | 98.0 | 10.5 | 12.4 | 118.6 | 20.6 | 40.3 | 195.4 | 63.3 | 101 | 159.5 | 30 | 50.5 | 168.3 | H(5, N = 49) = 2.2 | 0.81 |
| NW (#) | 1.8 | 0.8 | 45.3 | 1.8 | 0.8 | 41.1 | 1.5 | 1.4 | 90.2 | 1.6 | 0.4 | 28.6 | 2.3 | 0.6 | 24.7 | 2.6 | 1.6 | 61 | H(5, N = 46) = 4.3 | 0.50 |
| NW (min) | 15 | – | – | – | – | – | 24.0 | 25.1 | 104.6 | 10 | 7.1 | 70.7 | – | – | – | – | – | – | H(5, N = 8) = 0.00 | 1.00 |
| WU (h) | 4.7 | 1.2 | 25.1 | 5.8 | 3.4 | 59.7 | 5.6 | 1.7 | 29.7 | 6.4 | 2.6 | 39.9 | 6.5 | 1.4 | 21.8 | 5.2 | 1.9 | 36.4 | H(5, N = 48) = 4.98 | 0.42 |
BT, bedtime; SOL, duration of sleep onset latency; NW, night waking; WU, wake up time; V, percentage variation in mean; M, mean; SD, standard deviation.
Within the GD and GM groups separately, there were no differences in the sleep pattern across the six groups.
Step 2 & 3: Melatonin levels at Start of Treatment and when Reported Effective
From the total sample, 43 participants started with supervised melatonin intake, which was on average 1.7 mg (SD = 1.6 mg, min: 0.1–max: 5 mg) (https://www.researchgate.net/publication/306224642).
In 32 participants, an effective dose (i.e., no longer exhibiting sleep complaints) was found, which was on average 1.7 mg (SD = 2.2 mg, min: 0.1–max: 10 mg). There was no significant relationship between age and effective exogenous melatonin doses (Pearson correlation: −0.03, P = 0.87; Figure 1).
In few participants, melatonin levels were collected consistently at each of the assessment points or there was insufficient saliva collected when under treatment. A Wilcoxon matched pair test indicated that “effective” melatonin doses were statistically lower than melatonin doses at intake (Z = 2.2, P = 0.03, Figure 3). Figure 4 illustrates the proportional distribution of low, moderate, and high levels of melatonin at 9–10 AM, 12 PM, 2 PM, 4 PM, 6 PM within the total sample (n = 59) at the start of treatment, yet too few data were collected to pursue statistical analyses across groups.
Figure 3.
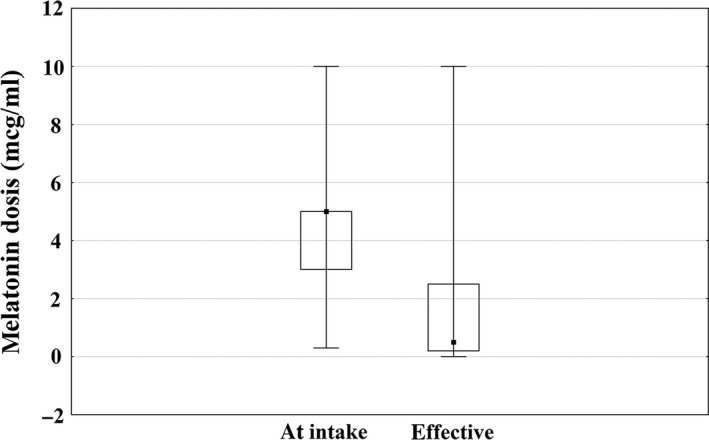
Melatonin doses at intake versus subjectively reported effective.
Figure 4.
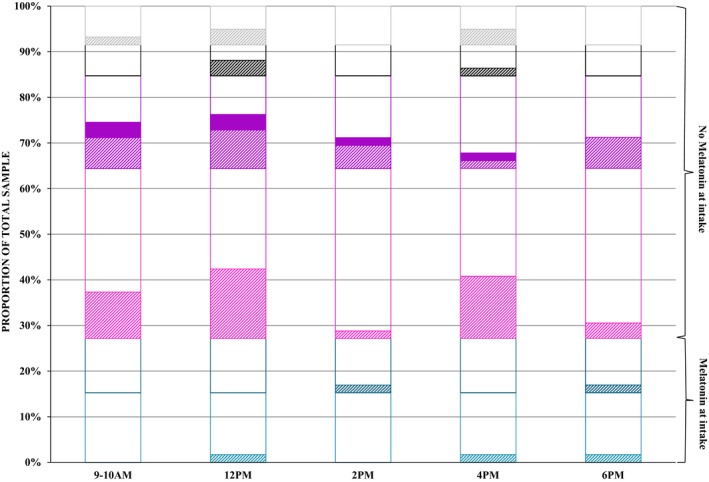
Proportional distribution of low, moderate, and high salivary melatonin levels of the total sample (six groups) under treatment. Salivary levels of melatonin: Dotted fill: <0.50pg/ml; striped fill: between 0.5–50pg/ml; complete fill: >50pg/ml; No fill: missing. Groups: Children ‐ Light cyan: boys taking melatonin at intake; dark cyan: girls taking melatonin at intake; light magenta: boys not taking melatonin at intake; dark magenta: girls not taking melatonin at intake; adults ‐ black: men not taking melatonin at intake and grey: women not taking melatonin at intake.
Other Medications that may affect the Metabolism of Melatonin
Co‐medication is not uncommon in samples with developmental disabilities; unfortunately, sample sizes were too small to investigate other medications in our analyses, and as a result, data will remain descriptive of the total sample (n = 59). At intake, four participants (one girl and one boy not on exogenous melatonin at intake, one girl and one boy on exogenous melatonin at intake) took 50 mg metoprolol. At the start of treatment, 22 subjects used metoprolol (M = 28 mg, SD = 61 mg; six girls and nine boys not on exogenous melatonin at intake, three girls and three boys on exogenous melatonin at intake, and one man not on exogenous melatonin at intake). When treatment was considered effective, in 15 participants, metoprolol dose was assessed (M = 37 mg, SD = 42 mg; three girls and seven boys not on exogenous melatonin at intake, two girls and two boys on exogenous melatonin at intake, and one man not on exogenous melatonin at intake).
In slow metabolizers of melatonin (https://www.researchgate.net/publication/307443362), exogenous melatonin may result in increasing daily melatonin levels. Melatonin is metabolized principally by the CYP1A2 enzyme. 25, 26, 27 Omeprazole, a strong inductor of CYP1A2 28, was administered to nine participants at start of treatment (M = 32 mg, SD = 12 mg; one girl and two boys not on exogenous melatonin at intake, two girls and 0 boy on exogenous melatonin at intake, and two women and two men not on exogenous melatonin at intake). These participants were CYP1A2 poor metabolizer, resulting in high melatonin levels after starting exogenous melatonin treatment. Omeprazole increases CYP1A2 levels 29 and was perceived enabling an effective treatment with exogenous melatonin, with less risk of 24‐h high melatonin levels.
Discussion
Overall, moderate to high levels of salivary melatonin at noon were found in individuals with SMS. In particular, children with SMS showed a disrupted melatonin pattern. Careful administration and titration of melatonin may be advocated. Furthermore, the endogenous melatonin level, age, and gender may potentially interact, yielding the severity range of sleep disturbances reported in SMS. Treatment of sleep problems in SMS is complex, and our findings may support person‐centered sleep and medication management. Future clinical trials including larger groups may shed light on such approaches.
Our findings corroborate previous study results in individuals with SMS 10, 12, 13, 14, 22, that is, individuals with SMS have disrupted levels of melatonin. More specifically, on the one hand, our findings add to the current knowledge that at noon, especially boys showed aberrant levels. Administration of melatonin to boys resulted in having proportionally fewer boys with moderate and more with high levels in comparison with others. On the other hand, our findings indicated that in the evening, primarily more girls on exogenous melatonin at intake exhibited high levels. Our results in girls concur with the finding that typically developing girls secrete more melatonin 19, 20. Corresponding to the gender, difference in melatonin secretion pattern is the large variation in sleep onset and offset problems in especially girls. Altogether, our findings may suggest that especially during childhood, the administration of melatonin as well as the level of endogenous melatonin needs to be monitored prior to embarking on a sleep intervention approach. Furthermore, they may elucidate the acknowledged complexity in the disrupted sleep rhythms in SMS.
Our findings regarding the melatonin level in combination with the caregiver‐reported sleep complaints are speculative toward melatonin metabolism. Braam et al. 26 recently reported in individuals with intellectual disabilities a loss of response to initially effective melatonin usage. The intriguing hypothesis that loss of response to melatonin treatment due to slow metabolism of exogenous melatonin may underpin Boone's et al.'s 22 speculation with respect to SMS. Inverted and delayed/advanced sleep phases have been phenotypically discussed in individuals with SMS. Noteworthy herein remains thus our finding regarding the potential age–gender interaction with respect to sleep rhythmicity. Indeed, participants reported in literature, in spite of the heterogeneity in their genotype, varied in terms of age and gender. This may have influenced their “disrupted” endogenous melatonin rhythmicity. Hypothetically, the accumulation of melatonin may auxiliarily impact their sleep–wake behavioral repertoire and rhythmicity. Given that daytime behavioral problems have been associated with increased melatonin levels 12, 13, 14, 22, 23, our findings may foster innovative pathways in dealing with the challenging behavior of individuals with SMS 30. Our finding of a significant lower than currently advised yet effective dosage of melatonin may further support this treatment approach.
About 27% (n = 16) of our clinical sample already took melatonin prior to consultation at the sleep clinic. Foremost all of them were in their childhood. Our findings underscore clinically three important notes. Firstly, high levels of melatonin can be expected in childhood 19. Yet a developmental sensitive and specific cutoff of melatonin level in children with SMS is undetermined. Secondly, difficulties falling asleep and maintaining sleep are top complaints in childhood 31, 32, 33. Furthermore, these complaints should be embedded within the socio‐emotional state of the child 34, 35, 36 and the caregiving context 37, 38, 39. Thirdly, researchers 3, 40 already advocated empirically based approaches to the use of both nonprescription and prescription medications for children with significant difficulties in initiating and/or maintaining sleep. We are unaware who promoted use of exogenous melatonin in our sample prior to consult at our clinic. Nonetheless, reflecting upon these three points in clinical practice is recommended as each may foster a multilayered sleep complaint within a disorder‐characteristic disrupted sleep rhythm.
To date, the timing of exogenous administration of melatonin as well as the type of exogenous melatonin might be considered as a potential confounder across studies. Also, the duration of treatment with melatonin could mask treatment results in light of the findings by Braam et al. (Braam et al., 2010). Hence, the matching of several treatment aspects such as beta‐blockers toward daytime somnolence and properly administered exogenous melatonin is known to be crucial for success in individuals with SMS 12, 14, 41, 42. In other words, before embarking on any type of management, given the acknowledged problems with the melatonin secretion pattern in SMS, a baseline needs to be established. In fact, personalized sleep medicine is an emerging area of research and practice 43.
There were methodological limitations in the present study that merit discussion. The sample was recruited from a sleep clinic. They all attended the clinic for the first time with sleep complaints characteristic of individuals with SMS. Most participants reach out to our clinic through parental organizations of children with developmental disabilities. For several reasons (e.g., ethical), we do not report whether they were self‐referred or provider‐referred, or whether melatonin was previously used but discontinued. The information regarding the duration of the sleep complaints or previous sleep treatments has not been reported in light of the early onset of sleep problems in individuals with SMS.
In addition, our current clinical practice is to collect saliva every 3 h toward a more successful data collection. For those taking melatonin at intake, levels reflect endogenous as well as exogenous melatonin. Aside from the doses of melatonin at intake, we did not analyze other details such as time of administration, which was set at 30 min. before bedtime under treatment. Given the average bedtime of the total sample, it would be around 18:57. Results are presented as a proportion of the total sample, that is, including missing data, which preserves the internal and external validity of the study, and as a result, findings reflect a sample of individuals with SMS having sleep complaints. We did not analyze any daytime behavioral problems or other nighttime problems such as parasomnias in this study.
To conclude, the severity range of sleep complaints encountered in individuals with SMS may vary substantially. Herein, the phenotypic characterization of problematic sleeping by age and gender might be of clinical relevance given their individual melatonin levels. Also, lower doses were found to be effective. Careful administration and titration of exogenous melatonin in relation to age, gender, and endogenous melatonin levels might be encouraged when sleep interventions are implemented in clinical practice. Alternatively, better understanding of treatment successes or failures, for example, beyond the “one pill for all” perspective, may provide opportunities to improve (sleep) behavior in those affected by developmental disabilities and their families. Given that all participants exhibited a sleep complaint, our findings may support person‐centered sleep and medication management.
Funding Source
No funding was secured for this study.
Financial Disclosure
The authors have no financial relationships relevant to this article to disclose.
Conflict of Interest
The authors declare no conflict of interest.
Ethical Approval and Informed Consent
All procedures were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. Informed consent was obtained from all individual participants included in the study.
Supporting information
Table S1. Mean and standard deviations of melatonin levels.
Acknowledgments
The authors would like to thank all patients and their families.
References
- 1. Cummings C; Canadian Paediatric Society CPC . Melatonin for the management of sleep disorders in children and adolescents. Paediatr Child Health 2012;17:331–336. [PMC free article] [PubMed] [Google Scholar]
- 2. Gringras P, Gamble C, Jones AP, et al. Melatonin for sleep problems in children with neurodevelopmental disorders: Randomised double masked placebo controlled trial. BMJ 2012;345:e6664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Schwichtenberg AJ, Malow BA. Melatonin treatment in children with developmental disabilities. Sleep Med Clin 2015;10:181–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bruni O, Alonso‐Alconada D, Besag F, et al. Current role of melatonin in pediatric neurology: Clinical recommendations. Eur J Paediatr Neurol 2015;19:122–133. [DOI] [PubMed] [Google Scholar]
- 5. Kennaway DJ. Potential safety issues in the use of the hormone melatonin in paediatrics. J Paediatr Child Health 2015;51:584–589. [DOI] [PubMed] [Google Scholar]
- 6. Appleton RE, Jones AP, Gamble C, et al. The use of melatonin in children with neurodevelopmental disorders and impaired sleep: A randomised, double‐blind, placebo‐controlled, parallel study (MENDS). Health Technol Assess 2012;16:i–239. [DOI] [PubMed] [Google Scholar]
- 7. Spruyt K, Curfs LM. Non‐pharmacological management of problematic sleeping in children with developmental disabilities. Dev Med Child Neurol 2015;57:120–136. [DOI] [PubMed] [Google Scholar]
- 8. Smith AC, McGavran L, Robinson J, et al. Interstitial deletion of (17)(p11.2p11.2) in nine patients. Am J Med Genet 1986;24:393–414. [DOI] [PubMed] [Google Scholar]
- 9. Slager RE, Newton TL, Vlangos CN, Finucane B, Elsea SH. Mutations in RAI1 associated with Smith‐Magenis syndrome. Nat Genet 2003;33:466–468. [DOI] [PubMed] [Google Scholar]
- 10. Smith AC, Dykens E, Greenberg F. Sleep disturbance in Smith‐Magenis syndrome (del 17 p11.2). Am J Med Genet 1998;81:186–191. [PubMed] [Google Scholar]
- 11. Dykens EM, Smith AC. Distinctiveness and correlates of maladaptive behaviour in children and adolescents with Smith‐Magenis syndrome. J Intellect Disabil Res 1998;6:481–489. [DOI] [PubMed] [Google Scholar]
- 12. De Leersnyder H, De Blois MC, Claustrat B, et al. Inversion of the circadian rhythm of melatonin in the Smith‐Magenis syndrome. J Pediatr 2001;139:111–116. [DOI] [PubMed] [Google Scholar]
- 13. De Leersnyder H. Inverted rhythm of melatonin secretion in Smith‐Magenis syndrome: From symptoms to treatment. Trends Endocrinol Metab [Review] 2006;17:291–298. [DOI] [PubMed] [Google Scholar]
- 14. De Leersnyder H, Claustrat B, Munnich A, Verloes A. Circadian rhythm disorder in a rare disease: Smith‐Magenis syndrome. Mol Cell Endocrinol 2006;2:88–91. [DOI] [PubMed] [Google Scholar]
- 15. De Leersnyder H. Smith‐Magenis syndrome. Handb Clin Neurol 2013;111:295–296. [DOI] [PubMed] [Google Scholar]
- 16. Tietze AL, Blankenburg M, Hechler T, et al. Sleep disturbances in children with multiple disabilities. Sleep Med Rev 2012;16:117–127. [DOI] [PubMed] [Google Scholar]
- 17. Potocki L, Glaze D, Tan DX, et al. Circadian rhythm abnormalities of melatonin in Smith‐Magenis syndrome. J Med Genet 2000;37:428–433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Lewy AJ. Clinical applications of melatonin in circadian disorders. Dialogues Clin Neurosci 2003;5:399–413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Crowley SJ, Acebo C, Carskadon MA. Human puberty: Salivary melatonin profiles in constant conditions. Dev Psychobiol 2012;54:468–473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Fideleff HL, Boquete H, Fideleff G, et al. Gender‐related differences in urinary 6‐sulfatoxymelatonin levels in obese pubertal individuals. J Pineal Res 2006;40:214–218. [DOI] [PubMed] [Google Scholar]
- 21. Chik CL, Rollag MD, Duncan WC, Smith AC. Diagnostic utility of daytime salivary melatonin levels in Smith‐Magenis syndrome. Am J Med Genet A 2010;152A:96–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Boone PM, Reiter RJ, Glaze DG, Tan DX, Lupski JR, Potocki L. Abnormal circadian rhythm of melatonin in Smith‐Magenis syndrome patients with RAI1 point mutations. Am J Med Genet A 2011;155A:2024–2027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Boudreau EA, Johnson KP, Jackman AR, et al. Review of disrupted sleep patterns in Smith‐Magenis syndrome and normal melatonin secretion in a patient with an atypical interstitial 17p11.2 deletion. Am J Med Genet A 2009;149A:1382–1391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Nagtegaal E, Peeters T, Swart W, Smits M, Kerkhof G, van der Meer G. Correlation between concentrations of melatonin in saliva and serum in patients with delayed sleep phase syndrome. Ther Drug Monit 1998;20:181–183. [DOI] [PubMed] [Google Scholar]
- 25. Braam W, Keijzer H, Struijker Boudier H, Didden R, Smits M, Curfs L. CYP1A2 polymorphisms in slow melatonin metabolisers: A possible relationship with autism spectrum disorder? J Intellect Disabil Res 2013;57:993–1000. [DOI] [PubMed] [Google Scholar]
- 26. Braam W, van Geijlswijk I, Keijzer H, Smits MG, Didden R, Curfs LM. Loss of response to melatonin treatment is associated with slow melatonin metabolism. J Intellect Disabil Res 2010;54:547–555. [DOI] [PubMed] [Google Scholar]
- 27. Zhou SF, Liu JP, Chowbay B. Polymorphism of human cytochrome P450 enzymes and its clinical impact. Drug Metab Rev 2009;41:89–295. [DOI] [PubMed] [Google Scholar]
- 28. Huuhka K, Riutta A, Haataja R, Ylitalo P, Leinonen E. The effect of CYP2C19 substrate on the metabolism of melatonin in the elderly: A randomized, double‐blind, placebo‐controlled study. Methods Find Exp Clin Pharmacol 2006;28:447–450. [DOI] [PubMed] [Google Scholar]
- 29. Han XM, Ouyang DS, Chen XP, et al. Inducibility of CYP1A2 by omeprazole in vivo related to the genetic polymorphism of CYP1A2. Br J Clin Pharmacol 2002;54:540–543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Hodapp RM, Fidler DJ, Smith AC. Stress and coping in families of children with Smith‐Magenis syndrome. J Intellect Disabil Res 1998;5(Pt 5):331–340. [DOI] [PubMed] [Google Scholar]
- 31. Spruyt K, O'Brien LM, Cluydts R, Verleye GB, Ferri R. Odds, prevalence and predictors of sleep problems in school‐age normal children. J Sleep Res 2005;14:163–176. [DOI] [PubMed] [Google Scholar]
- 32. Li L, Ren J, Shi L, et al. Frequent nocturnal awakening in children: Prevalence, risk factors, and associations with subjective sleep perception and daytime sleepiness. BMC Psychiatry 2014;14:204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Carter KA, Hathaway NE, Lettieri CF. Common sleep disorders in children. Am Fam Physician 2014;89:368–377. [PubMed] [Google Scholar]
- 34. Fernandez‐Mendoza J, Vgontzas AN, Calhoun SL, et al. Insomnia symptoms, objective sleep duration and hypothalamic‐pituitary‐adrenal activity in children. Eur J Clin Invest 2014;44:493–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Turnbull K, Reid GJ, Morton JB. Behavioral sleep problems and their potential impact on developing executive function in children. Sleep 2013;36:1077–1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Alvaro PK, Roberts RM, Harris JK. A Systematic review assessing bidirectionality between sleep disturbances, anxiety, and depression. Sleep 2013;36:1059–1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Wiggs L, Stores G. Behavioural treatment for sleep problems in children with severe learning disabilities and challenging daytime behaviour: Effect on sleep patterns of mother and child. J Sleep Res 1998;7:119–126. [DOI] [PubMed] [Google Scholar]
- 38. Wiggs L, Stores G. Behavioural treatment for sleep problems in children with severe intellectual disabilities and daytime challenging behaviour: Effect on mothers and fathers. Br J Health Psychol 2001;6:257–269. [DOI] [PubMed] [Google Scholar]
- 39. Stores G, Wiggs L. Sleep Disturbance in Children and Adolescents with Disorders of Development: Its Significance and Management. London, UK: Mac Keith Press, 2001. [Google Scholar]
- 40. Owens JA, Rosen CL, Mindell JA. Medication use in the treatment of pediatric insomnia: Results of a survey of community‐based pediatricians. Pediatrics 2003;1:e628–e635. [DOI] [PubMed] [Google Scholar]
- 41. Carpizo R, Martinez A, Mediavilla D, Gonzalez M, Abad A, Sanchez‐Barcelo EJ. Smith‐Magenis syndrome: A case report of improved sleep after treatment with beta1‐adrenergic antagonists and melatonin. J Pediatr 2006;149:409–411. [DOI] [PubMed] [Google Scholar]
- 42. D Leersnyder e H, Bresson JL, de Blois MC, et al. Beta 1‐adrenergic antagonists and melatonin reset the clock and restore sleep in a circadian disorder, Smith‐Magenis syndrome. J Med Genet 2003;40:74–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Smits M, Keijzer H, Braam W, Vervoort J, Curfs L. Personalized sleep medicine applied to melatonin treatment for circadian rhythm sleep disorders: Current status and future. Sleep Med 2013;14:e271. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Mean and standard deviations of melatonin levels.


