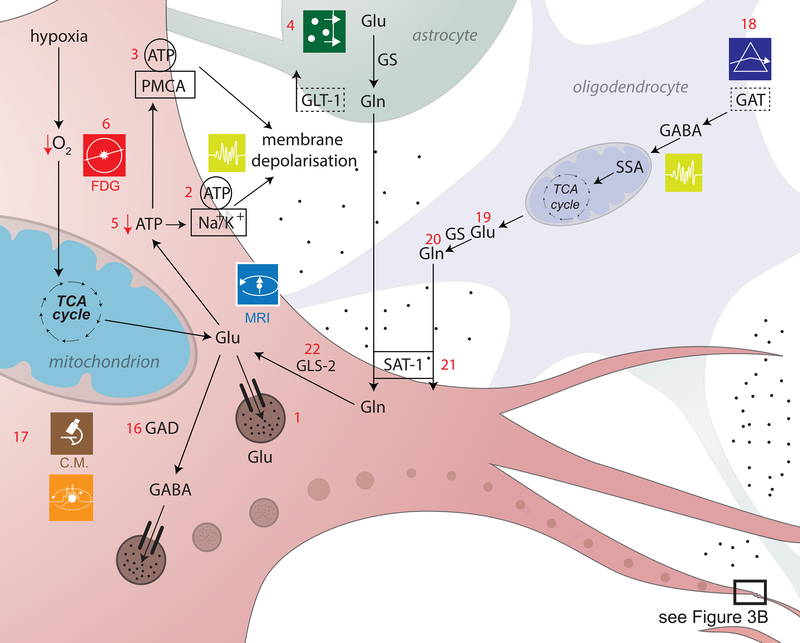
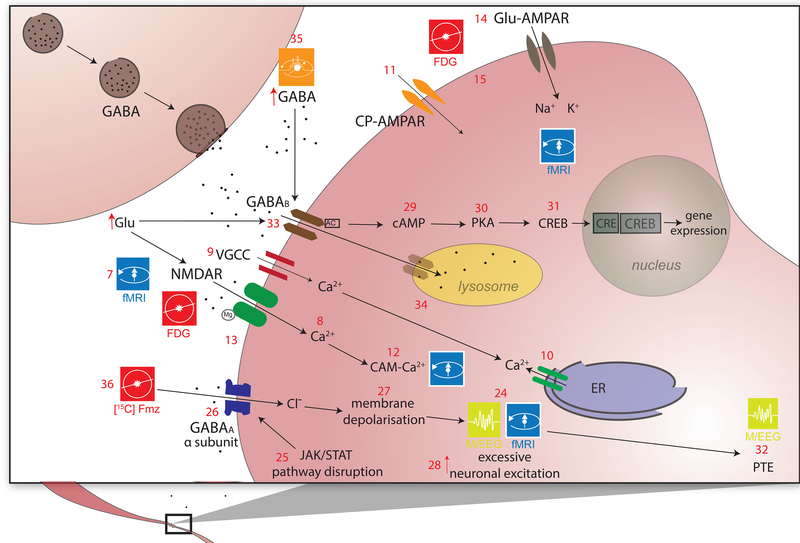
Figure 3a,b. Excitotoxicity and inhibitory dysregulation.
In this figure, 3b is a magnified illustration of the synaptic level shown in 3a. In 3a, Glu is the biochemical compound with the highest concentration in the mammalian brain[137]. It is the carboxylate anion of glutamic acid, a non-essential α-amino acid used extensively for protein biosynthesis. Glu is the primary excitatory neurotransmitter in the CNS, where it activates neurons via its dedicated receptors[138]. Within cells, Glu is typically stored in vesicles (3a.1), while extracellular Glu is recycled by plasma membrane transporters at a steady rate to avoid its accumulation in the extracellular space[139]. Since ATP must bind both to the Na+/K+ (sodium/potassium) ATPase (3a.2) and to the plasma membrane Ca2+ ATPase (PMCA) (3a.3) to maintain the electrochemical potential of the cell membrane, TBI-related hypoxia results in ATP production impairment, and thereby to membrane depolarization. In 3b, the accumulation of extracellular Glu results in the opening of an excessive fraction of Ca2+-permeable Glu NMDARs (3b.7) and in the consequent flow of Ca2+ into the neuron (3b.8)[140],[141]. Simultaneously, a membrane potential decrease due to NMDAR activation results in the opening of voltage-gated Ca2+ channels (VLCCs) (3b.9), which leads to further membrane potential decrease. γ-aminobutyric acid (GABA) is produced from Glu by means of the tonically-active glutamic acid decarboxylase (GAD) enzyme (3a.16). In the mammalian CNS, it is the chief neurotransmitter responsible for inhibiting synaptic activity[46, 47], exerting its effect primarily via the activation of ionotropic GABAA receptors. GABA concentrations are relatively higher presynaptically, where its levels are controlled by phasically-active GAD[48]. Synaptically-released GABA is uptaken by glia via GABA transporters (GATs) (3a.18) and converted to Glu in mitochondria (3a.19). Glu is then converted to glutamine (Gln) (3a.20) and transported by system A Gln transporters (SATs) back into neurons (3a.21), where Gln is reconverted to Glu, by the action of glutaminase (GLS-2) (3a.22) and reused to produce GABA[142]. Subsequent to TBI, the number of GATs decreases partly due to the death of GABAergic neurons, where GATs are produced. This lowers the rate at which GABA is reuptaken by glia, such that the extracellular concentration of GABA increases and the rate of its conversion to Glu and then to Gln decreases[143],[144]. The net result of this process is a decrease in the amount of Gln which is exported from glia to the extracellular space and then to GABAergic neurons via SATs[145]. After TBI, GABAergic signaling may be disrupted partly because of modifications to GABAA subunits, including the GABAA α1, α4, γ2 and δ subunits (3b.26). The earliest changes to the α1 subunit occur in the first day after injury and are brought about by disruptions of the pathway involving the Janus Kinase (JaK) and Signal Transducer and Activator of Transcription (STAT) protein (i.e. the JAK/STAT pathway) (3b.25)[146],[147],[148]. Such disruptions result in neuronal membrane depolarization (3b.27) and in weaker downregulation of neuronal excitation (3b.28)[149]. GABAB regulates gene expression by activating protein kinase A (PKA) (3b.30) in the presence of cyclic adenosine monophosphate (cAMP). This second messenger is bound to by cAMP (3b.29) response element-binding (CREB) proteins (3b.31), thereby increasing or decreasing the transcription of downstream genes (3b.32)[150].