Summary
Aquaporin 4 (AQP4) is the main water channel in the central nervous system (CNS) and specifically localized to astrocyte processes. Recent studies indicate that AQP4 regulates various biological functions of astrocytes, including maintaining CNS water balance, spatial buffering of extracellular potassium, calcium signal transduction, regulation of neurotransmission, synaptic plasticity, and adult neurogenesis, while under neuropathological conditions, AQP4 has a role in astrogliosis and proinflammatory cytokine secretion. In addition, accumulating evidence suggests that, besides cerebral edema, neuromyelitis optica and epilepsy, AQP4 participates in the onset and progression of Alzheimer disease, Parkinson disease, depression, and drug addiction. This review summarizes recent findings and highlights the involvement of AQP4 in astrocyte function and neuropsychiatric disorders.
Keywords: Aquaporin 4, Astrocytes, Central nervous system, Neuropsychiatric disorders
Introduction
Aquaporins (AQP), also known as water channels, are widely expressed on cell membranes and integral for transporting water and other small molecules, such as glycerine, selectively and efficiently 1. To date, at least 13 AQP members have been found in mammals 1. In the central nervous system (CNS), three water channels (AQP1, AQP4, and AQP9) have been identified 2. AQP1 is mainly localized to the apical membrane of choroid plexus epithelial cells and involved in the secretion of cerebrospinal fluid 3, 4. AQP9 expression is found in tanycytes mainly localized in the circumventricular regions and catecholaminergic neurons in the hindbrain and implicated in brain energy metabolism 5, 6. AQP4 is the predominant water channel in the CNS and distinctively expressed in astrocyte processes and the basolateral cell plasma membrane of ependymal cells throughout the brain and spinal cord 7, 8. Great progress has been made on the biological characteristics and functions of AQP4 in the CNS, compared with the relatively limited studies on AQP1 and AQP9.
Here, we review literature regarding the involvement of AQP4 in the biological function of astrocytes and the pathophysiological processes of neuropsychiatric disorders.
Regulating Astrocyte Function
Astrocytes are the most abundant glial cells in the mammal brain and responsible for a wide variety of essential functions in the physiology and pathology of the CNS. They maintain brain fluid, iron, and transmitter homeostasis, regulate neural signal transduction, and secret several neural growth factors, such as brain‐derived neural factor (BDNF) and glial‐derived neural factor (GDNF) 9, 10. Moreover, astrocytes undergo variable degrees of activation (reactive astrogliosis) and produce proinflammatory cytokines in respond to all forms of brain injury and disease 11.
In mice, the functions and biological features of astrocytes are altered upon AQP4 gene deletion. Evidence for the involvement of AQP4‐based rapid water transport in astrocyte function is summarized below.
Establishment and Maintenance of the CNS Water Homeostasis
Astrocytes regulate water exchange between the brain, blood, and within brain compartments through AQP4 12. AQP4 knockout (AQP4−/−) mice were shown to exhibit slightly increased baseline water content in the brain and spinal cord, compared with wild‐type (AQP4+/+) littermates 13, 14, 15. AQP4 also plays a key role in the water uptake of the brain after birth. Early studies reported that AQP4 expression levels in the rat cerebellum were extremely low in the first postnatal week, but significantly increased in the second week 16. More recent studies revealed that increased AQP4 expression levels partially relate to decreased brain water content in postnatal mice 17, 18. Furthermore, a significant delayed decrease in brain water content occurs in the newborn systemic or conditional AQP4−/− mice, providing direct evidence for a role of AQP4 in postnatal brain water uptake 18, 19.
Potassium Spatial Buffering
Regulating neuronal electrical activity is one of the important physiological functions of astrocytes; the process involves spatial buffering and uptake of extracellular K+ 20. During the repolarization process, K+ channels on the neuronal cell membrane open, causing the concentration of K+ to increase within the extracellular space. Astrocytes take up excess K+ through the inward rectifier potassium channel (Kir 4.1). K+ is then redistributed through the astroglial syncytium via gap junctions, thereby stabilizing neuronal activity 21. AQP4 gene deletion resulted in delayed water transport coupling extracellular K+ clearance, which subsequently affected neuroexcitation. This view is supported by the phenotype analysis of AQP4−/− mice that showed impaired vision 22, hearing 23 and olfaction 24, as well as reduced seizure threshold, increased seizure duration, and slowed K+ kinetics 25, 26, 27 (Figure 1).
Figure 1.
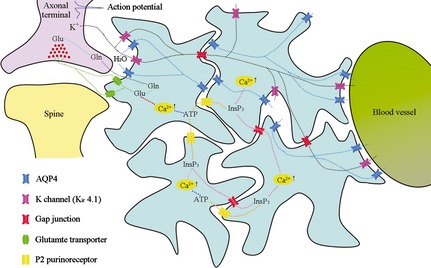
AQP4 involvement in the regulation of CNS water, iron, and glutamate homeostasis and glial calcium wave propagation. (1) Potassium is released into the extracellular space (ECS) during repolarization of the action potential. Astrocyte processes surrounding the synapse take up excess K+ through inward rectifier potassium channels Kir 4.1 (local potassium buffering), redistribute the K+ through the astroglial syncytium via gap junctions (spatial potassium buffering), and release K+ through Kir 4.1. (2) Glutamate released during synaptic activity is removed from the ECS mainly by glutamate transporters on the astrocyte processes. After entering the astrocytes, glutamate is converted into glutamine, which is then transported back to the presynaptic terminal for synthesizing glutamate. The glutamate–glutamine shuttle allows for sustained glutamatergic synaptic transmission and prevents postsynaptic neurons from glutamate excitotoxicity. (3) Intracellular glutamate can also activate calcium signals in astrocyte processes enwrapping synaptic contacts. Calcium signals propagate through the astroglial syncytium via ATP‐P2 purinoreceptor‐inositol trisphosphate (InsP3) pathway. (4) AQP4 facilitates water entry into astrocyte processes surrounding the synapse, transports water through the astroglial network, and releases distantly into the ECS surrounding microvessels. The AQP4‐mediated rapid transport of intercellular water would drive reuptake of K+ and glutamate and Ca2+ signaling transduction by astrocytes, because water serves as transport medium for these substances.
Calcium Signal Transduction
Ca2+ signaling is a characteristic form of cellular excitability that serves as a mediator of bidirectional interactions between neurons and astrocytes 9. Astrocytic Ca2+ signaling also plays a role in neuronal death following ischemia 28. Now there is evidence for a critical role of AQP4 in astrocyte Ca2+ signal transduction. Using in vivo two‐photon imaging, Thrane and colleagues demonstrated that conditional deletion of AQP4 reduced hypoosmotic stress‐evoked Ca2+ signaling in astrocytes 29. Their in vitro experiments further suggested that AQP4‐dependent Ca2+ signal transduction is mediated in part by autocrine purinergic signaling 29. Apart from affecting the pathological outcome of cerebral edema, AQP4‐dependent Ca2+ signals may mediate astrocyte–astrocyte or astrocyte–neuronal communication in normal physiological conditions, hereby providing a sophisticated means for information exchange in the CNS (Figure 1).
Regulation of Neurotransmission
Glutamate is the most prominent neurotransmitter in the brain. Astrocytes mediate glutamate uptake by excitatory amino acid receptors 30. Glutamate uptake is also accompanied by water transport, which can cause astrocyte processes to swell around the synapses, resulting in a reduction in the extracellular synaptic space during synaptic transmission and processing 31. To restore extracellular space volume, astrocytes transport water further into the surrounding capillary via AQP4 located in the perivascular endfeet (Figure 1). Both in vitro and in vivo evidence indicates that AQP4 gene deletion in mice downregulates glutamate transporter 1 expression and impairs glutamate uptake ability 32, 33, 34, 35. Previous studies have also suggested an involvement of AQP4 in the metabolism of dopamine, serotonin, and other neurotransmitters 36, 37.
Adult Neurogenesis
Adult neurogenesis mainly occurs in the subventricular zone (SVZ) of the lateral cerebral ventricle and the subgranular zone (SGZ) of the dentate gyrus, where a large number of neural stem/progenitor cells reside 38. In both locations, astroglia are the stem elements that produce neurons. These “stem” astrocytes differ from “classical” mature astrocytes by radial morphology, specific expression of the protein nestin, and for some astrocytes, the formation of cilia 39. In vitro experiments showed that AQP4 gene deletion in mice impaired proliferation, migration, and neuronal differentiation of adult neural stem cells 40. The deletion also disrupted fluoxetine treatment‐induced adult mouse hippocampal neurogenesis under both basal and chronic mild stress‐evoked depressive conditions 41. In physiological conditions, adult AQP4−/− mice showed altered neurogenesis in SVZ, but not in SGZ, compared with AQP4+/+ controls 41. The discrepant roles of AQP4 in adult SGZ and SVZ neurogenesis may be due to the different microenvironments; further studies are necessary to explore the underlying mechanisms.
Neurotrophin‐dependent Synaptic Plasticity
Astrocytes mediate synaptic plasticity via secretion of neurotrophic factors such as BDNF and GDNF 9. Recent studies have shown that AQP4 is involved in the regulation of neurotrophic factor‐dependent synaptic plasticity. AQP4−/− mice demonstrate impaired BDNF‐dependent long‐term potentiation (LTP) 42 and long‐term depression (LTD), which could be rescued by a scavenger of BDNF or blockade of Trk receptors 42. In addition, AQP4 gene deletion in mice was shown to exacerbate 1‐methyl‐4‐phenyl‐1,2,3,6‐tetrahydropyridine (MPTP)‐induced dopaminergic degeneration associated with inhibited astroglial proliferation and GDNF protein synthesis 43. Adult AQP4−/− mice exhibited defects in consolidation memory and location‐specific object memory 42, 44, 45, which is consistent with impaired neurotrophin‐dependent synaptic plasticity. These findings highlight that AQP4 has a role in synaptic plasticity and cognitive function, although the exact mechanisms warrant further investigation.
Astrocyte Migration and Reactivation
Reactive astrogliosis and glial scar formation are hallmarks of all brain injuries and diseases and therefore may exert a number of essential beneficial functions in response to CNS insults 11. Astrocyte migration toward the lesion is the key step toward glial scar formation and is regulated by various factors, including growth factors, cytokines, and mediators of innate immunity 46. There is compelling evidence from in vitro and in vivo studies that suggest a critical role for AQP4 in astrocyte migration. Compared with AQP4+/+ controls, primary cultured astrocytes from AQP4−/− mice were shown to have similar morphology, adhesion, and proliferation, but significantly impaired migration ability in the wound healing assay and the transwell Boyden chamber assay 47. Consistent with these results, using the mouse cortical stab injury model, implanted AQP4−/− astrocytes, prelabeled with a fluorescent dye, showed greatly impaired migration toward the injured site 48. AQP4‐mediated astrocyte migration may facilitate water influx across lamellipodia at the leading edge of a migrating cell and promote membrane protrusion, although the exact mechanism remains unclear 49, 50. AQP4 gene deletion in mice appeared to inhibit astrocyte proliferation, reactivation, and scar formation in severe traumatic brain and spinal cord injuries 48, 51, and in chemical agent‐induced neurodegeneration 43, 52, 53. Taken together, these results suggest that AQP4 is a unique target for regulating astrocyte activation in various CNS disorders and pathologies.
Secretion of Proinflammatory Cytokines
In response to different kinds of stimulation, reactive astrocytes can exert either pro‐ or antiinflammatory potential, which is determined by context‐specific signaling mechanisms 46. In vitro cultured studies have shown that lipopolysaccharide (LPS)‐induced TNF‐alpha and IL‐6 secretion was reduced in AQP4−/− astrocytes, while increased in adenovirus transfected AQP4 overexpression astrocytes 54. Likewise, attenuated neuroinflammation was observed in AQP4−/− mice following intracerebral injection of LPS 54. These results demonstrate the role of AQP4 in promoting cytokine secretion from reactive astrocytes. However, other studies have shown AQP4 gene deletion in mice to increase secretion of proinflammatory factors in the heart following treatment with a β‐receptor agonist, isoproterenol, resulting in the aggravation of cardiac failure and arrhythmias 55. These discrepant results indicate that AQP4 plays different proinflammatory roles in different pathological conditions. The mechanism still requires further analysis.
Involvement in Neuropsychiatric Disorders
Reactive astrocytes play either a primary or contributing role in CNS disorders, either via loss of normal astrocyte functions or gain of abnormal effects 56. Protection of astrocyte function has been implicated as a far more effective strategy than direct protection of neurons in neurological disorders 10. Thus, identifying molecular targets for functional regulation of astrocytes and elucidating their therapeutic potential have important theoretical and practical significance. There are several in‐depth, extensive reviews on the role of AQP4 in astrocyte dysfunction under various neuropathological conditions, such as stroke, cerebral edema, neuromyelitis optica, and epilepsy 57, 58, 59, 60, 61. Here, we only summarize the pathophysiological roles of neurodegenerative diseases including Alzheimer disease (AD), Parkinson disease (PD), and psychiatric disorders, such as depression and drug addiction.
Alzheimer Disease
Alzheimer disease is the most common neurodegenerative disease among the elderly and characterized by beta‐amyloid (Aβ) plaque deposition, neurofibrillary tangles, and neuronal and synapse loss in learning and memory related regions 62. Recent studies have suggested that AQP4 is an important functional regulator on astrocyte plasticity and involved in the progression of AD. Altered AQP4 expression in astrocytes has been observed in the brain tissues of patients with AD and several AD models, for example, upregulated expression around Aβ plaques, or loss of expression from endfoot membranes at vascular amyloids 63, 64, 65, 66. In vitro experiments have shown that Aβ 1–42 increases AQP4 expression in cultured mouse cortical astrocytes when present at low concentrations, but decreases AQP4 expression when at high concentrations. AQP4 gene deletion reduces Aβ 1–42‐induced astrocyte activation and apoptosis, which is associated with a reduction in the uptake of Aβ via decreased upregulation of low‐density lipoprotein‐receptor‐related protein‐1 67. In vivo studies on APP/PS1 transgenic AD model mice further revealed that AQP4 gene deletion impairs exogenous Aβ clearance from brain parenchyma 68 and exacerbates spatial learning and memory defects associated with more severe Aβ plaque deposits and synaptic protein loss (unpublished data). These in vitro and in vivo results highlight that AQP4 can be considered a molecular target for Aβ metabolism and clearance in AD.
Parkinson Disease
Parkinson disease is another common neurodegenerative disease that generally strikes middle‐aged adults. To date, there are several hypotheses on the pathogenesis of PD, of which include the neuroinflammatory hypothesis 69. Evidence from several recent studies indicates an involvement of AQP4 in the onset and progression of PD. AQP4 mRNA levels were shown to be significantly downregulated in the plasma of PD patients, compared with normal subjects 70. AQP4 gene deletion caused severe dopaminergic neuronal loss and microglial inflammation induced by systemic MPTP injection 43. Further studies revealed that AQP4 deficiency decreases CD4(+) CD25(+) regulatory T cells, resulting in hyperactive immune responses, potentially contributing to the increased severity of PD 71. The specific mechanism of AQP4 in PD pathogenesis warrants further investigation.
Depression
Increasing evidence indicates that adult hippocampal neurogenesis plays a key role in the pathogenesis of depression 72. Fluoxetine is a common antidepressant drug, and its therapeutic action is mainly via promoting hippocampal neurogenesis 73. As mentioned earlier, AQP4 gene deletion suppresses fluoxetine‐induced hippocampal neurogenesis in the CMS mouse model of depression 41. In vitro experiments also showed that AQP4 deficiency inhibits fluoxetine‐induced proliferation of cultured NSCs 41. AQP4 might therefore serve as a new target for antidepressant therapy.
Addiction
Addiction is a state of periodic or chronic intoxication produced by the repeated consumption of a drug or the practice of a harmful activity. Studies have reported that cocaine‐induced addiction mainly results from alterations in dopaminergic neurotransmission from the ventral tegmental area to the nucleus accumbens 74, 75, 76. AQP4−/− mice have shown to exhibit reduced spontaneous activity and extracellular dopamine levels in nucleus accumbens following exposure to cocaine 77. Repeated cocaine administration significantly decreased cellular proliferation in the hippocampal SGZ, and AQP4 gene deletion resisted this reduction. Further studies have suggested that AQP4 is involved in the negative regulation of neurogenesis by cocaine via PKC‐mediated signal transduction 78.
Concluding Remarks
Accumulating evidence supports the notion that glial water channel AQP4 not only plays important physiological functions in the normal CNS, but also participates in a variety of neuropsychiatric disorders. AQP4 may serve as a functional regulator of astrocytes for the treatment of CNS diseases or injuries. AQP4 regulates multiple functions of astrocytes and is also extensively expressed in peripheral organs and tissues; thus, the potential side effects of AQP4 drugs, such as agonists, antagonists, as well as opening or blocking agents, should be noted for treatment of CNS diseases associated with astrocyte dysfunction.
Conflict of Interest
The authors declare no conflict of interest.
Acknowledgements
This work was supported by grants from the National Nature and Scientific Foundation of China (No. 81271210).
References
- 1. Agre P, King LS, Yasui M, et al. Aquaporin water channels‐from atomic structure to clinical medicine. J Physiol 2002;542:3–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Papadopoulos MC, Verkman AS. Aquaporin water channels in the nervous system. Nat Rev Neurosci 2013;14:265–277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Oshio K, Watanabe H, Song Y, Verkman AS, Manley GT. Reduced cerebrospinal fluid production and intracranial pressure in mice lacking choroid plexus water channel Aquaporin‐1. FASEB J 2004a;19:76–78. [DOI] [PubMed] [Google Scholar]
- 4. Boassa D, Yool AJ. Physiological roles of aquaporins in the choroid plexus. Curr Top Dev Biol 2005;67:181–206. [DOI] [PubMed] [Google Scholar]
- 5. Badaut J, Regli L. Distribution and possible roles of aquaporin 9 in the brain. Neuroscience 2004;129:971–981. [DOI] [PubMed] [Google Scholar]
- 6. Rojek AM, Skowronski MT, Füchtbauer EM, et al. Defective glycerol metabolism in aquaporin 9 (AQP9) knockout mice. Proc Natl Acad Sci USA 2007;104:3609–3614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Nielsen S, Nagelhus EA, Amiry‐Moghaddam M, Bourque C, Agre P, Ottersen OP. Specialized membrane domains for water transport in glial cells: High‐resolution immunogold cytochemistry of aquaporin‐4 in rat brain. J Neurosci 1997;17:171–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Oshio K, Binder DK, Yang B, Schecter S, Verkman AS, Manley GT. Expression of aquaporin water channels in mouse spinal cord. Neuroscience 2004b;127:685–693. [DOI] [PubMed] [Google Scholar]
- 9. Allen NJ, Barres BA. Neuroscience: Glia‐more than just brain glue. Nature 2009;457:675–677. [DOI] [PubMed] [Google Scholar]
- 10. Barres BA. The mystery and magic of glia: A perspective on their roles in health and disease. Neuron 2008;60:430–440. [DOI] [PubMed] [Google Scholar]
- 11. Sofroniew MV, Vinters HV. Astrocytes: Biology and pathology. Acta Neuropathol 2010;119:7–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Amiry‐Moghaddam M, Ottersen OP. The molecular basis of water transport in the brain. Nat Rev Neurosci 2003;4:991–1001. [DOI] [PubMed] [Google Scholar]
- 13. Bloch O, Auguste KI, Manley GT, Verkman AS. Accelerated progression of kaolin‐induced hydrocephalus in aquaporin‐4‐deficient mice. J Cereb Blood Flow Metab 2006;26:1527–1537. [DOI] [PubMed] [Google Scholar]
- 14. Li X, Kong H, Wu W, Xiao M, Sun X, Hu G. Aquaporin‐4 maintains ependymal integrity in adult mice. Neuroscience 2009;162:67–77. [DOI] [PubMed] [Google Scholar]
- 15. Saadoun S, Bell BA, Verkman AS, Papadopoulos MC. Greatly improved neurological outcome after spinal cord compression injury in AQP4‐deficient mice. Brain 2008;131:1087–1098. [DOI] [PubMed] [Google Scholar]
- 16. Wen H, Nagelhus EA, Amiry‐Moghaddam M, Agre P, Ottersen OP, Nielsen S. Ontogeny of water transport in rat brain: Postnatal expression of the aquaporin‐4 water channel. Eur J Neurosci 1999;11:935–945. [DOI] [PubMed] [Google Scholar]
- 17. Ferrari DC, Nesic OB, Perez‐Polo JR. Oxygen resuscitation does not ameliorate neonatal hypoxia/ischemia‐induced cerebral edema. J Neurosci Res 2010;88:2056–2065. [DOI] [PubMed] [Google Scholar]
- 18. Li X, Gao J, Ding J, Hu G, Xiao M. Aquaporin‐4 expression contributes to decreases in brain water content during mouse postnatal development. Brain Res Bull 2013;94:49–55. [DOI] [PubMed] [Google Scholar]
- 19. Haj‐Yasein NN, Vindedal GF, Eilert‐Olsen M, et al. Glial‐conditional deletion of aquaporin‐4 (Aqp4) reduces blood‐brain water uptake and confers barrier function on perivascular astrocyte endfeet. Proc Natl Acad Sci USA 2011;108:17815–17820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kofuji P, Newman E. Potassium buffering in the central nervous system. Neuroscience 2004;129:1043–1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Butt AM, Kalsi A. Inwardly rectifying potassium channels (Kir) in central nervous system glia: A special role for Kir4.1 in glial functions. J Cell Mol Med 2006;10:33–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Li J, Patil RV, Verkman AS. Mildly abnormal retinal function in transgenic mice without Muller cell aquaporin‐4 water channels. Invest Ophthalmol Vis Sci 2002;43:573–579. [PubMed] [Google Scholar]
- 23. Li J, Verkman AS. Impaired hearing in mice lacking aquaporin‐4 water channels. J Biol Chem 2001;276:31233–31237. [DOI] [PubMed] [Google Scholar]
- 24. Lu DC, Zhang H, Zador Z, Verkman AS. Impaired olfaction in mice lacking aquaporin‐4 water channels. FASEB J 2008;22:3216–3223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Binder DK, Oshio K, Ma T, Verkman AS, Manley GT. Increased seizure threshold in mice lacking aquaporin 4 water channels. NeuroReport 2004;15:259–262. [DOI] [PubMed] [Google Scholar]
- 26. Binder DK, Yao X, Zador Z, Sick TJ, Verkman AS, Manley GT. Increased seizure duration and slowed potassium kinetics in mice lacking aquaporin‐4 water channels. Glia 2006;53:631–636. [DOI] [PubMed] [Google Scholar]
- 27. Jin BJ, Zhang H, Binder DK, Verkman AS. Aquaporin‐4‐dependent K(+) and water transport modeled in brain extracellular space following neuroexcitation. J Gen Physiol 2013;141:119–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Bano D, Nicotera P. Ca2 + signals and neuronal death in brain ischemia. Stroke 2007;38:674–676. [DOI] [PubMed] [Google Scholar]
- 29. Thrane AS, Rappold PM, Fujita T, et al. Critical role of aquaporin‐4 (AQP4) in astrocyte Ca2 + signaling events elicited by cerebral edema. Proc Natl Acad Sci USA 2011;108:846–851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. O'Shea RD. Roles and regulation of glutamate transporters in the central nervous system. Clin Exp Pharmacol Physiol 2002;29:1018–1023. [DOI] [PubMed] [Google Scholar]
- 31. Gunnarson E, Zelenina M, Axehult G, et al. Identification of a molecular target for glutamate regulation of astrocyte water permeability. Glia 2008;56:587–596. [DOI] [PubMed] [Google Scholar]
- 32. Zeng XN, Sun XL, Gao L, Fan Y, Ding JH, Hu G. Aquaporin‐4 deficiency down‐regulates glutamate uptake and GLT‐1 expression in astrocytes. Mol Cell Neurosci 2007;34:34–39. [DOI] [PubMed] [Google Scholar]
- 33. Wu N, Lu XQ, Yan HT, et al. Aquaporin 4 deficiency modulates morphine pharmacological actions. Neurosci Lett 2008;448:221–225. [DOI] [PubMed] [Google Scholar]
- 34. Li YK, Wang F, Wang W, et al. Aquaporin‐4 deficiency impairs synaptic plasticity and associative fear memory in the lateral amygdala: Involvement of downregulation of glutamate transporter‐1 expression. Neuropsychopharmacology 2012;37:1867–1878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Yan HT, Wu N, Lu XQ, Su RB, Zheng JQ, Li J. Aquaporin‐4 deficiency attenuates opioid dependence through suppressing glutamate transporter‐1 down‐regulation and maintaining glutamate homeostasis. CNS Neurosci Ther 2013;19:12–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Fan Y, Zhang J, Sun XL, et al. Sex‐ and region‐specific alterations of basal amino acid and monoamine metabolism in the brain of aquaporin‐4 knockout mice. J Neurosci Res 2005;82:458–464. [DOI] [PubMed] [Google Scholar]
- 37. Ding JH, Sha LL, Chang J, Zhou XQ, Fan Y, Hu G. Alterations of striatal neurotransmitter release in aquaporin‐4 deficient mice: An in vivo microdialysis study. Neurosci Lett 2007;422:175–180. [DOI] [PubMed] [Google Scholar]
- 38. Alvarez‐Buylla A, García‐Verdugo JM, Tramontin AD. A unified hypothesis on the lineage of neural stem cells. Nat Rev Neurosci 2001;2:287–293. [DOI] [PubMed] [Google Scholar]
- 39. Verkhratsky A, Butt A. Glial Neurobiology. West Sussex, UK: John Wiley & Sons Ltd, 2007. [Google Scholar]
- 40. Kong H, Fan Y, Xie J, et al. AQP4 knockout impairs proliferation, migration and neuronal differentiation of adult neural stem cells. J Cell Sci 2008;121:4029–4036. [DOI] [PubMed] [Google Scholar]
- 41. Kong H, Sha LL, Fan Y, et al. Requirement of AQP4 for antidepressive efflciency of fluoxetine: Implication in adult hippocampal neurogenesis. Neuropsychopharmacology 2009;34:1263–1276. [DOI] [PubMed] [Google Scholar]
- 42. Skucas VA, Mathews IB, Yang J, et al. Impairment of select forms of spatial memory and neurotrophin‐dependent synaptic plasticity by deletion of glial aquaporin‐4. J Neurosci 2011;31:6392–6397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Fan Y, Kong H, Shi X, et al. Hypersensitivity of aquaporin 4‐deficient mice to 1‐methyl‐4‐phenyl‐1,2,3,6‐tetrahydropyrindine and astrocyte modulation. Neurobiol Aging 2008;29:1226–1236. [DOI] [PubMed] [Google Scholar]
- 44. Fan Y, Liu M, Wu X, et al. Aquaporin‐4 promotes memory consolidation in Morris water maze. Brain Struct Funct 2013;218:39–50. [DOI] [PubMed] [Google Scholar]
- 45. Zhang J, Li Y, Chen ZG, et al. Glia protein aquaporin‐4 regulates aversive motivation of spatial memory in Morris water maze. CNS Neurosci Ther 2013;19:937–944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Sofroniew MV. Molecular dissection of reactive astrogliosis and glial scar formation. Trends Neurosci 2009;32:638–647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Saadoun S, Papadopoulos MC, Watanabe H, Yan D, Manley GT, Verkman AS. Involvement of aquaporin‐4 in astroglial cell migration and glial scar formation. J Cell Sci 2005;118:5691–5698. [DOI] [PubMed] [Google Scholar]
- 48. Auguste KI, Jin S, Uchida K, Yan D, Manley GT, Papadopoulos MC. Greatly impaired migration of implanted aquaporin‐4‐deficient astroglial cells in mouse brain toward a site of injury. FASEB J 2007;21:108–116. [DOI] [PubMed] [Google Scholar]
- 49. Papadopoulos MC, Saadoun S, Verkman AS. Aquaporins and cell migration. Pflugers Arch 2008;456:693–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Loitto VM, Karlsson T, Magnusson KE. Water flux in cell motility: Expanding the mechanisms of membrane protrusion. Cell Motil Cytoskeleton 2009;66:237–247. [DOI] [PubMed] [Google Scholar]
- 51. Wu Q, Zhang YJ, Gao JY, et al. Aquaporin‐4 mitigates retrograde degeneration of rubrospinal neurons by facilitating edema clearance and glial scar formation after spinal cord injury in mice. Mol Neurobiol [Epub ahead of print] Doi: 10.1007/s12035-013-8607-3. [DOI] [PubMed] [Google Scholar]
- 52. Lu DC, Zador Z, Yao J, Fazlollahi F, Manley GT. Aquaporin‐4 reduces post‐traumatic seizure susceptibility by promoting astrocyte glial scar formation in mice. J Neurotrauma 2011. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Liu L, Lu Y, Hu G, et al. Aquaporin‐4 deficiency exacerbates brain oxidative damage and memory deficits induced by long‐term ovarian hormone deprivation and D‐galactose injection. Int J Neuropsychopharmacol 2012;15:55–68. [DOI] [PubMed] [Google Scholar]
- 54. Li L, Zhang H, Varrin‐Doyer M, Zamvil SS, Verkman AS. Proinflammatory role of aquaporin‐4 in autoimmune neuroinflammation. FASEB J 2011;25:1556–1566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Cheng YS, Tang YQ, Dai DZ, Dai Y. AQP4 knockout mice manifest abnormal expressions of calcium handling proteins possibly due to exacerbating pro‐inflammatory factors in the heart. Biochem Pharmacol 2012;83:97–105. [DOI] [PubMed] [Google Scholar]
- 56. Seifert G, Schilling K, Steinhäuser C. Astrocyte dysfunction in neurological disorders: A molecular perspective. Nat Rev Neurosci 2006;7:194–206. [DOI] [PubMed] [Google Scholar]
- 57. Papadopoulos MC, Verkman AS. Aquaporin‐4 and brain edema. Pediatr Nephrol 2007;22:778–784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Zador Z, Stiver S, Wang V, Manley GT. Role of aquaporin‐4 in cerebral edema and stroke. Handb Exp Pharmacol 2009;190:159–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Jarius S, Paul F, Franciotta D, et al. Mechanisms of disease: Aquaporin‐4 antibodies in neuromyelitis optica. Nat Clin Pract Neurol 2008;4:202–214. [DOI] [PubMed] [Google Scholar]
- 60. Verkman AS, Ratelade J, Rossi A, Zhang H, Tradtrantip L. Aquaporin‐4: Orthogonal array assembly, CNS functions, and role in neuromyelitis optica. Acta Pharmacol Sin 2011;32:702–710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Binder DK, Nagelhus EA, Ottersen OP. Aquaporin‐4 and epilepsy. Glia 2012;60:1203–1214. [DOI] [PubMed] [Google Scholar]
- 62. Goedert M, Spillantini MG. A century of Alzheimer's disease. Science 2006;314:777–781. [DOI] [PubMed] [Google Scholar]
- 63. Moftakhar P, Lynch MD, Pomakian JL, Vinters HV. Aquaporin expression in the brains of patients with or without cerebral amyloid angiopathy. J Neuropathol Exp Neurol 2010;69:1201–1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Wilcock DM, Vitek MP, Colton CA. Vascular amyloid alters astrocyte water and potassium channels in mouse models and humans with Alzheimer's disease. Neuroscience 2009;159:1055–1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Hoshi A, Yamamoto T, Shimizu K, et al. Characteristics of aquaporin expression surrounding senile plaques and cerebral amyloid angiopathy in Alzheimer disease. J Neuropathol Exp Neurol 2012;71:750–759. [DOI] [PubMed] [Google Scholar]
- 66. Yang J, Lunde LK, Nuntagij P, et al. Loss of astrocyte polarization in the tg‐ArcSwe mouse model of Alzheimer's disease. J Alzheimers Dis 2011;27:711–722. [DOI] [PubMed] [Google Scholar]
- 67. Yang W, Wu Q, Yuan C, et al. Aquaporin‐4 mediates astrocyte response to β‐amyloid. Mol Cell Neurosci 2012;49:406–414. [DOI] [PubMed] [Google Scholar]
- 68. Iliff JJ, Wang M, Liao Y, et al. A paravascular pathway facilitates CSF flow through the brain parenchyma and the clearance of interstitial solutes, including amyloid β . Sci Transl Med 2012;4:147ra111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Greene JG. Current status and future directions of gene expression profiling in Parkinson's disease. Neurobiol Dis 2012;45:76–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Thenral ST, Vanisree AJ. Peripheral assessment of the genes AQP4, PBP and TH in patients with Parkinson's disease. Neurochem Res 2012;37:512–515. [DOI] [PubMed] [Google Scholar]
- 71. Chi Y, Fan Y, He L, et al. Novel role of aquaporin‐4 in CD4 + CD25 + T regulatory cell development and severity of Parkinson's disease. Aging Cell 2011;10:368–382. [DOI] [PubMed] [Google Scholar]
- 72. Eisch AJ, Petrik D. Depression and hippocampal neurogenesis: A road to remission? Science 2012;338:72–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Santarelli L, Saxe M, Gross C, Surget A, Battaglia F, Dulawa S. Requirement of hippocampal neurogenesis for the behavioral effects of antidepressants. Science 2003;301:805–809. [DOI] [PubMed] [Google Scholar]
- 74. Thomas MJ, Beurrier C, Bonci A, Malenka RC. Long‐term depression in the nucleus accumbens: A neural correlate of behavioral sensitization to cocaine. Nat Neurosci 2001;4:1217–1223. [DOI] [PubMed] [Google Scholar]
- 75. Steketee JD. Cortical mechanisms of cocaine sensitization. Crit Rev Neurobiol 2005;17:69–86. [DOI] [PubMed] [Google Scholar]
- 76. Zhang Y, Loonam TM, Noailles PA, Angulo JA. Comparison of cocaine‐ and methamphetamine‐evoked dopamine and glutamate overflow in somatodendritic and terminal field regions of the rat brain during acute, chronic, and early withdrawal conditions. Ann N Y Acad Sci 2001;937:93–120. [DOI] [PubMed] [Google Scholar]
- 77. Li Z, Gao L, Liu Q, et al. Aquaporin‐4 knockout regulated cocaine‐induced behavior and neurochemical changes in mice. Neurosci Lett 2006;403:294–298. [DOI] [PubMed] [Google Scholar]
- 78. Xie LL, Sun XL, Fan Y, Kong H, Ding JH, Hu G. Aquaporin 4 knockout resists negative regulation of neural cell proliferation by cocaine in mouse hippocampus. Int J Neuropsychopharmacol 2009;12:843–850. [DOI] [PubMed] [Google Scholar]


