Abstract
Background
Psoriasis is a chronic skin disease that may develop at any age. Estimates for the United States and Europe suggest that psoriasis accounts for 4% of skin diseases in children. In most cases, the condition is mild and can be treated with creams. However, a small percentage of children have moderate to severe disease that requires drugs, such as ciclosporin or methotrexate, and some will require injections with newer biological agents, such as anti‐TNF (tumour necrosis factor) drugs. Anti‐TNF drugs (among them etanercept, infliximab, and adalimumab) are designed to reduce inflammation in the body caused by tumour necrosis factor. Evidence for the safety and efficacy of these biological agents in paediatric psoriasis is lacking.
Objectives
To assess the efficacy and safety of anti‐TNF agents for the treatment of paediatric psoriasis.
Search methods
We searched the following databases up to July 2015: the Cochrane Skin Group Specialised Register, the Cochrane Central Register of Controlled Trials (CENTRAL; 2015, Issue 6), MEDLINE (from 1946), Embase (from 1974), and LILACS (from 1982). We also searched 13 trials registers and checked the reference lists of included studies and key review articles for further references to relevant randomised controlled trials (RCTs). We handsearched conference proceedings and attempted to contact trial authors and relevant pharmaceutical manufacturers. We searched the US Food and Drug Administration's and European Medicines Agency's adverse effects databases.
Selection criteria
All relevant RCTs that evaluated the efficacy and safety of anti‐TNF agents for the treatment of chronic plaque psoriasis in individuals less than 18 years of age.
Data collection and analysis
Two review authors independently checked titles and abstracts and performed data extraction and 'Risk of bias' assessment of the included studies. One review author entered data into Review Manager (RevMan), and a second review author checked the data. We also attempted to obtain unclear data from the trial authors where possible.
Our primary outcomes were investigator‐assessed number of participants achieving a 75% improvement in Psoriasis Area and Severity Index‐75 (PASI 75) compared to baseline, improvement in quality of life using an instrument such as Children's Dermatology Life Quality Index (CDLQI), and adverse effects. Our secondary outcomes included the proportion of participants achieving PASI 50 and the Physician's Global Assessment (PGA).
Main results
We included one study with 211 participants (median age 13 years), in which etanercept (dosage ranged from 0.8 to 50 mg per kilogram of body weight) was compared to placebo. Follow‐up was over a 48‐week period.
At week 12, 57% versus 11% who received etanercept or placebo, respectively, achieved the PASI 75 (risk ratio 4.95, 95% confidence interval (CI) 2.83 to 8.65; high‐quality evidence). Absolute risk reduction and the number needed to treat to obtain a benefit with etanercept was 45% (95% CI 33.95 to 56.40) and 2 (95% CI 1.77 to 2.95), respectively.
The percentage improvement from baseline of the CDLQI scores at week 12 was better in the etanercept group than the placebo group (52.3% versus 17.5%, respectively (P = 0.0001)). Analysis between the groups showed an effect size that was clinically important (mean difference 2.30, 95% CI 0.85 to 3.75; high‐quality evidence). However, means, medians, and minimal important difference results and results of the Pediatric Quality of Life Inventory, Stein Impact on Family Scale, and Harter Self‐Perception Profile for Children scores must be interpreted with caution, as they were not prespecified outcomes.
Three serious adverse events were reported, but they were resolved without sequelae. Deaths or other events such as malignant tumours, opportunistic infections, tuberculosis, or demyelination were not reported in the included study.
Also, 13% of participants in the placebo group and 53% in the etanercept group had a PGA of clear or almost clear (risk ratio 3.96, 95% CI 2.36 to 6.66; high‐quality evidence) at week 12.
Authors' conclusions
This review found only one RCT evaluating the use of this type of biological therapy. Although the risk of publication bias was high, as we included only one industry‐sponsored RCT, the risk of allocation, selection, performance, attrition, and selective reporting biases for all outcomes (except for CDLQI) was low, and no short‐term serious adverse events were found.
We can conclude, based on this single included study, that etanercept seems to be efficacious and safe (at least in the short term) for the treatment of paediatric psoriasis. However, as the GRADE approach refers not to individual studies but to a body of evidence, we shall wait for the results of the ongoing studies in a future update of this review. In addition, future studies should evaluate quality‐of‐life endpoints established a priori and standardise primary outcome measures such as PASI 75, and should include the PGA as a secondary endpoint. Also, collating and reporting adverse events uniformly is required to better evaluate safety.
Plain language summary
Anti‐TNF agents for paediatric psoriasis
Background
Psoriasis is a long‐term skin disease that may develop at any age. Estimates for the United States and Europe suggest that psoriasis accounts for 4% of skin diseases in children. In most cases, the condition is mild and can be treated with creams. However, a small percentage of children have moderate to severe disease that requires drugs, such as ciclosporin or methotrexate, and some will require injections with newer biological agents, such as anti‐TNF (tumour necrosis factor) drugs. Anti‐TNF drugs (among them etanercept, infliximab, and adalimumab) are designed to reduce inflammation in the body caused by tumour necrosis factor.
Review question
Are anti‐TNF drugs such as etanercept, infliximab, and adalimumab safe and effective for treating moderate to severe psoriasis in children under 18 years of age?
Study characteristics
We searched for all randomised controlled trials (RCTs) that assessed the efficacy and safety of anti‐TNF agents for the treatment of long‐term plaque psoriasis in individuals younger than 18 years of age. We searched databases up to July 2015. Only one study (with three phases: a 12‐week randomised, double‐blind, placebo‐controlled phase; a 24‐week open‐label phase, and a 12‐week phase of a randomised, double‐blind, withdrawal–retreatment design) investigating one anti‐TNF agent (etanercept) in 211 participants met the inclusion criteria.
Key results
Evidence from this single included study suggests that by week 12 etanercept reduced the extent of the psoriasis in children when compared with placebo. Although a few adverse events were reported, they were resolved without subsequent problems. We did not find any evidence on long‐term side effects of this drug from this included study.
Quality of the evidence
Although this one RCT provided high‐quality evidence for the Physician's Global Assessment and all Psoriasis Area and Severity Index scores (75, 90, and 50) and moderate‐quality evidence for quality‐of‐life outcomes, we found no further randomised studies either evaluating etanercept or comparing other anti‐TNF agents, highlighting the need for further well‐designed randomised studies involving the use of biological therapies in children and young people with psoriasis. Several studies are ongoing that have not yet been completed or published. We plan to include the results of these in future updates of this review.
Summary of findings
Summary of findings for the main comparison. Etanercept compared to placebo for paediatric psoriasis.
| Etanercept compared to placebo for paediatric psoriasis | ||||||
| Patient or population: people with paediatric psoriasis Settings: multicentre Intervention: etanercept Comparison: placebo | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | Number of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Placebo | Etanercept | |||||
| PASI 75 Participants achieving a 75% improvement in the Psoriasis Area and Severity Index Follow‐up: 12 weeks | 114 per 1000 | 566 per 1000 (323 to 989) | RR 4.95 (2.83 to 8.65) | 211 (1 study) | ⊕⊕⊕⊕ high | As this review assessed the quality of evidence of just 1 individual trial, a greater body of evidence is needed to make a full recommendation |
| PASI 50 Participants achieving a 50% improvement in the Psoriasis Area and Severity Index Follow‐up: 12 weeks | 229 per 1000 | 745 per 1000 (517 to 1000) | RR 3.26 (2.26 to 4.71) | 211 (1 study) | ⊕⊕⊕⊕ high | As this review assessed the quality of evidence of just 1 individual trial, a greater body of evidence is needed to make a full recommendation |
| PASI 90 Participants achieving a 90% improvement in the Psoriasis Area and Severity Index Follow‐up: 12 weeks | 67 per 1000 | 273 per 1000 (125 to 597) | RR 4.10 (1.88 to 8.95) | 211 (1 study) | ⊕⊕⊕⊕ high | As this review assessed the quality of evidence of just 1 individual trial, a greater body of evidence is needed to make a full recommendation |
| PGA Participants achieving a PGA of 'clear' or 'almost clear' Follow‐up: 12 weeks | 133 per 1000 | 528 per 1000 (315 to 888) | RR 3.96 (2.36 to 6.66) | 211 (1 study) | ⊕⊕⊕⊕ high | As this review assessed the quality of evidence of just 1 individual trial, a greater body of evidence is needed to make a full recommendation |
| CDLQI Scores range from 0 to 30. Lower scores indicate better health‐related quality of life. Calculations are based on the change from baseline to week 12 in both groups Follow‐up: 12 weeks | ‐ | The mean CDLQI in the intervention groups was 2.30 higher (0.85 to 3.75 higher) | ‐ | 211 (1 study) | ⊕⊕⊕⊝ moderate1 | As this review assessed the quality of evidence of just 1 individual trial, a greater body of evidence is needed to make a full recommendation |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CDLQI: Children's Dermatology Life Quality Index; CI: Confidence interval; PASI: Psoriasis Area and Severity Index; PGA: Physician's Global Assessment; RR: Risk ratio | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 Downgraded one level due to selective reporting of means, medians, and the minimal important difference, as they were not prespecified in the methods section of the original study.
Background
Description of the condition
Psoriasis is a chronic skin disease that may develop at any age. The prevalence of condition is estimated to range from 0.5% to 3.8% worldwide (Augustin 2010; de Jager 2010a; Seyhan 2006; Tollefson 2010). A United Kingdom study of both adults and children and a paediatric population study in the United States demonstrated an incidence of 140 per 100,000 and 40.8 per 100,000, respectively (Huerta 2007; Tollefson 2010). It has been suggested that the incidence of psoriasis in children and adults may be increasing over time (Tollefson 2010). Approximately one‐third of people with psoriasis developed the condition before 20 years of age (Ferrándiz 2002; Tollefson 2010). Psoriasis in childhood and adolescence differs from the onset of psoriasis in adulthood, which has led to the concept of 'paediatric onset psoriasis' (POP) in contrast to 'adult onset psoriasis' (AOP) (Raychaudhuri 2000; Sticherling 2011). As in adults, in children chronic plaque psoriasis is the most common subtype, accounting for between 30% and 60% of the reported types in children. It is common for the scalp and face to be affected when it presents. Compared with adults, guttate psoriasis is more frequent in children, as is flexural or inverse psoriasis, often affecting the genital area (Benoit 2007; Leman 2001; Sticherling 2011).
The pathogenesis of psoriasis is still not fully understood, but it is thought to develop in genetically predisposed individuals, since 23.4% to 71% of children have a family history of psoriasis (Silverberg 2009). An environmental trigger, such as a streptococcal infection (Burden 1999; Farber 1999; Marji 2010), has been also described. Psoriasis is a T‐cell‐mediated disease with elevated levels of pro‐inflammatory cytokines, such as interleukin‐17 (IL‐17) (Chiricozzi 2011; Harper 2009), interferon‐γ (IFN‐γ) (Cai 2012; Victor 2003), tumour necrosis factor‐alpha (TNF‐α) (Victor 2003), IL‐22, and IL‐23 (Cai 2012; Chiricozzi 2014). Blocking the effects of TNF‐α with biological therapies has been shown to be therapeutically effective in moderate to severe psoriasis (Blandizzi 2014; Victor 2003).
There is currently no universally agreed outcome measures to assess severity and extent of disease in a paediatric population. By default, the Psoriasis Area and Severity Index (PASI) has been adopted as a measure of disease extent in clinical trials, although its use has never been validated in children (Spuls 2010). In this index, lesion characteristics and affected area are included in a formula that results in a score from 0 to 72 (Spuls 2010), but it is less accurate in younger children due to differences in body surface area (BSA) (Langley 2011).
Other tools used for the assessment of psoriasis in children are the Patient Global Assessment, the seven‐point Psoriasis Global Assessment score (Paller 2008; Paller 2010a), and BSA, which is based on the "rule of nines" (the head and neck, each arm, the front and back of each leg, as well as the four trunk quadrants each cover 9% of body surface area with the genitalia covering the final 1% of the body surface area) (Ramsay 1991; Spuls 2010).
The impact of psoriasis on children and their families is less well documented than in adults, but data are emerging in this area showing that there is significant disease impact in these people and their families, mainly due to the associated symptoms of joint pain and itch (Gånemo 2011). A quality of life (QOL) study on 379 children and adolescents aged between 5 and 16 years found QOL impairment scores in psoriasis that were higher than in children with acne or urticaria and similar to children with atopic dermatitis (Beattie 2006).
Regarding the relationship between PASI and PGA and health‐related quality of life outcomes in children, only a moderate correlation between PASI and PGA severity scores and Children's Dermatology Quality of Life Index (CDLQI) in paediatric psoriasis was found (de Jager 2010b).
Also, for children with psoriasis, there are limited supporting data for associated comorbidities, such as cardiovascular disease. In recent years, hypertension has been found to be increased in childhood psoriasis (Augustin 2010).
Most psoriasis in children can be easily managed with topical therapies. However, moderate to severe disease can be more difficult to manage due to licensing limitations and the lack of efficacy data related to the use of standard systemic agents and the newer biological therapies (Sticherling 2011; Vogel 2012).
Children with more extensive lesions often require short courses of phototherapy or systemic therapies. The commonly used oral therapies in childhood (as in adults) are ciclosporin, methotrexate, and acitretin (de Jager 2010a). Clinical studies of the use of ciclosporin in childhood psoriasis are scarce; only case reports have been described (Sticherling 2011).
The use of ciclosporin is justified for those with moderate to severe disease, however adequate renal function and blood pressure monitoring must be carried out (Sticherling 2011). Methotrexate is available for longer term use in the paediatric population (Sticherling 2011). Clinical double‐blind studies exist for juvenile idiopathic arthritis, and psoriatic arthritis rather than psoriasis. Studies on the treatment of psoriasis in childhood are lacking, and only individual case reports have been published. In addition, acitretin can be used to treat psoriasis in children, but its effects on epiphysial closure and on bone growth limit its use (Sticherling 2011).
The newer biological therapies may be beneficial for the treatment of psoriasis in children. Evidence is required to assess the efficacy of these agents and their side effects in a paediatric population.
Description of the intervention
A biologic or biotechnology‐derived product is defined as "a protein or nucleic acid–based pharmaceutical substance used for therapeutic or in vivo diagnostic purposes, which is produced by means other than direct extraction from a native (nonengineered) biological source" (Walsh 2002). Recently, it has been shown that children with moderate to severe psoriasis, which is poorly controlled with other treatments, may benefit from biologic therapies described below (Sticherling 2011). Currently, there are no US Food and Drug Administration (FDA)‐approved systemic treatments for moderate to severe psoriasis in children and adolescents. However, an etanercept study in children over eight years of age resulted in the licensing of the medication in Europe for children over this age (Sticherling 2011).
The anti‐TNF class of drugs includes etanercept, infliximab, and adalimumab, which act to down‐regulate the biological effect of the cytokine TNF‐α, which has been shown to be integral in the pathogenesis of chronic plaque psoriasis (Fallon‐Friedlander 2002; Krueger 2004).
These biological therapies have FDA approval for the treatment of other inflammatory disorders affecting children four years of age or older, such as rheumatoid and psoriatic arthritis and Crohn's disease (Fallon‐Friedlander 2002; Krueger 2004).
Whilst all these therapies block the biological effects of TNF‐α (Krueger 2004), they are different drugs.
The first of these agents to gain FDA approval, infliximab, is a chimeric monoclonal antibody that binds to TNF‐α, blocking the binding to receptors, with the consequent inactivation of the inflammatory process, which is thought to play a key role in the pathogenesis of psoriasis (Menter 2004). It is administered via intravenous infusion, and although licensed for use in psoriasis in adults, it is not currently licensed for use in children. Nevertheless, its use in children older than six years of age has been licensed for therapy‐refractory Crohn's disease (Menter 2004; Sticherling 2011).
Adalimumab is a fully human monoclonal antibody that binds to TNF‐α, blocking its interaction with cell surface TNF receptors (Lapadula 2014). It is licensed only for adults for the treatment of chronic plaque psoriasis. Its use in children older than four years of age has been licensed for polyarticular juvenile idiopathic arthritis (Menter 2004; Sticherling 2011). In 2015, adalimumab was licensed for the treatment of severe chronic plaque psoriasis in children older than four years of age (EMA 2015).
Etanercept is a fusion protein that binds to the extracellular domain of the human TNF‐α receptor, inhibiting the binding of TNF‐α to cell surface receptors. It is administered twice weekly by subcutaneous injection (Fallon‐Friedlander 2002; Krueger 2004). It currently has FDA approval for juvenile idiopathic arthritis in children as young as four years of age and was approved in 2002 for psoriatic arthritis. It is the only TNF‐α agent licensed for use in the United Kingdom and Europe for children from eight years of age with moderate psoriasis (Fallon‐Friedlander 2002; Krueger 2004). A randomised controlled trial (RCT) of etanercept administered to children for 12 weeks and subsequent long‐term data at 96 weeks suggest that the drug is beneficial for psoriasis and appears to be well tolerated (Paller 2008; Paller 2010a).
In addition to direct TNF‐α blocking agents, there are other biologics that interfere indirectly with TNF‐α cellular release, such as agents targeting T‐cells or antigen‐presenting cells (alefacept and efalizumab), IL‐12/IL‐23 blockers (ustekinumab, briakinumab), and phosphodiesterase‐4 inhibitors (apremilast) (Elliott 2009; Gordon 2012; Schafer 2012; Weger 2010). Recent emerging biologics for psoriasis (ixekizumab, brodalumab) have also focused on the inhibition of IL‐17, which acts synergistically with TNF‐α (Chiricozzi 2011; Leonardi 2012; Papp 2012).
How the intervention might work
Tumour necrosis factor‐α is an inflammatory cytokine that orchestrates the inflammatory response and the production of adhesion molecules (for example intercellular adhesion molecule 1, P‐selectin, E‐selectin) and pro‐inflammatory molecules (for example IL‐1, IL‐6, IL‐8, NF‐kappaB) (Victor 2003). The anti‐TNF therapies described reduce the biological activity of this cytokine by down‐regulating the stimulated pathways of keratinocyte proliferation and cell adhesion, which drive psoriasis (Krueger 2002; Mittal 2010).
The immune system of some people with psoriasis does not respond to treatment with TNF‐blocking agents. This is called primary treatment failure. Other individuals may respond initially, with the treatment becoming less effective, demonstrating secondary treatment failure (Puig 2013). Finally, there is also a paradoxical 'triggering' of psoriasis in a subgroup of people treated with anti‐TNF agents for conditions such as rheumatoid arthritis and Crohn's disease (Iborra 2011).
Why it is important to do this review
Children with moderate to severe psoriasis require adequate treatment, and this cannot always be achieved with the conventional systemic therapies described above. Individuals with severe and recalcitrant disease may benefit from biological therapies, which are still relatively new, high‐cost drugs each estimated to cost around GBP 10,000 per person per year (NICE 2012).
Evidence is required to assess the efficacy and safety of systemic therapies for children in general, but particularly for these newer biological therapies for which the long‐term side effects are unknown. The short‐term side effects of these drugs in adults are increasingly well understood and include reactions at the injection site, allergic reactions, and re‐activation of infections, particularly tuberculosis (Marji 2010). Also, such long‐term effects of these drugs as lymphoma in children have been described (McCroskery 2010). It is therefore important to evaluate the short‐ and long‐term adverse effects of these agents when they are used to treat children with psoriasis.
In this systematic review we looked at the evidence for the efficacy and safety of a subset of biological therapies in children, namely the anti‐TNF agents.
We published the plans for this review as a protocol: Anti‐TNF agents for paediatric psoriasis (Sanclemente 2012).
Objectives
To assess the efficacy and safety of anti‐tumour necrosis factor (anti‐TNF) agents for the treatment of paediatric psoriasis.
Methods
Criteria for considering studies for this review
Types of studies
All RCTs that evaluated the efficacy and safety of anti‐TNF agents for chronic plaque psoriasis treatment in individuals younger than 18 years of age.
Types of participants
In this review, we included any children (under 18 years old) with a clinical or histopathological diagnosis of chronic plaque psoriasis. This included types of psoriasis where there was an indication for the use of anti‐TNF agents, that is children with moderate to severe plaque psoriasis who did not respond to, had a contraindication to, or who did not tolerate other systemic therapies, including ciclosporin, methotrexate, or photochemotherapy using psoralen (PUVA).
Types of interventions
Any anti‐TNF agent (or any agent that acts to block the biological activity of TNF‐α) at any dosage, administered either orally, subcutaneously, or intravenously, either alone or in combination with additional agents. The considered comparators were:
any alternative active treatment (PUVA, narrow‐band ultraviolet B, acitretin, methotrexate, ciclosporin A, or any other biologic);
placebo; or
no treatment.
Types of outcome measures
Primary outcomes
Investigator‐assessed improvement: proportion of participants achieving PASI 75. (If this scale was not available, we planned to use PASI 50 or PASI 90) (see Differences between protocol and review).
Improvement in quality of life: assessed using a recognised instrument (generic, dermatology‐specific (such as CDLQI), Pediatric Quality of Life Inventory, or a disease‐specific instrument).
Proportion of participants having any minor or major adverse outcomes.
Secondary outcomes
Proportion of participants achieving PASI 50, PASI 90, or both.
Investigator‐assessed improvement: PGA.
Investigator‐assessed improvement: affected BSA.
Participant‐assessed improvement: Patient Global Assessment.
Psoriasis remission, recurrence, and resource use.
Timing of outcome assessment: We planned to consider main and secondary endpoint data (PASI 50, PASI 90, PGA, BSA, and adverse events) at less than three months, between three months and one year, and after one year. We grouped these into short, medium, and long term, according to how they were assessed in the trials.
Search methods for identification of studies
We aimed to identify all relevant RCTs regardless of language or publication status (published, unpublished, in press, or in progress).
Electronic searches
We searched the following databases up to 20 July 2015:
the Cochrane Skin Group Specialised Register using the search strategy in Appendix 1;
the Cochrane Central Register of Controlled Trials (CENTRAL) 2015, Issue 6, in the Cochrane Library using the strategy in Appendix 2;
MEDLINE via OVID (from 1946) using the strategy in Appendix 3;
Embase via OVID (from 1974) using the strategy in Appendix 4; and
LILACS (Latin American and Caribbean Health Science Information database, from 1982) using the strategy in Appendix 5.
Trials registers
We searched the following trials registers on 9 July 2015, using the search terms in Appendix 6:
The metaRegister of Controlled Trials (www.controlled‐trials.com).
The US National Institutes of Health ongoing trials register (www.clinicaltrials.gov).
The Australian New Zealand Clinical Trials Registry (www.anzctr.org.au).
The World Health Organization International Clinical Trials Registry Platform (www.who.int/trialsearch).
The ISRCTN Register (http://isrctn.org).
Netherlands Trial Register (http://www.trialregister.nl/).
UMIN Clinical Trials Registry (UMIN‐CTR) (http://www.umin.ac.jp/ctr).
NIH Clinical Research Studies (http://clinicalstudies.info.nih.gov).
Chinese Clinical Trial Registry (http://www.chictr.org.cn/index.aspx).
Clinical Trials Registry ‐ India ( http://ctri.nic.in/Clinicaltrials/advancesearchmain.php ).
Registro Público Cubano de Ensayos Clínicos (http://rpcec.sld.cu/).
Registro Nacional de Ensayos Clínicos del Peru (http://www.ins.gob.pe).
The Latin American Clinical Trial Registry (http://www.latinrec.org), but found it to be currently inactive.
Searching other resources
References from included studies
We scanned the bibliographies of included and other important studies and key review articles for further references to relevant trials.
Unpublished literature
We corresponded with the following pharmaceutical companies in order to obtain information about unpublished or ongoing trials: Abbott and Amgen Inc (in 2002 Immunex was acquired by Amgen Inc). In the United States, etanercept (Enbrel®) has been co‐marketed by Amgen and Pfizer, and Wyeth (which is part of Pfizer) was its sole marketer outside the United States excluding Japan. We corresponded with the following pharmaceutical companies, which we believe market or distribute anti‐TNF therapies, in order to obtain information about unpublished or ongoing trials: Abbott; Amgen Inc; Pfizer; Wyeth (which is part of Pfizer); UCB Inc; Janssen‐Cilag Ltd, which is now a subsidiary of Johnson & Johnson; UCB S.A.; Merck Serono; and Janssen Biotech, Inc.
Conference proceedings
We scanned the abstracts of the American Academy of Dermatology from February 2011 through to March 2015, and the European Academy of Dermatology and Venereology meetings from September 2011 through to September 2014. We also scanned the abstracts from the 23rd World Congress of Dermatology held in Vancouver, Canada, in June 2015.
Adverse effects
We reviewed the US Food and Drug Administration quarterly reports from April 2008 through to June 2015 without the use of a specific search term. We consulted the European Medicines Agency reports up to June 2015 to obtain safety data (see search strategy in Appendix 7).
We also considered adverse and side effects described in our included and excluded studies.
Data collection and analysis
Selection of studies
Two review authors (GS and JC) checked the titles and abstracts identified from the searches. After reviewing the abstracts, they retrieved the full text of potentially relevant studies for assessment. Both review authors independently assessed if, from reading the full text, each study met the predefined selection criteria. A third review author (RM) was available to resolve any differences through discussion. We have listed excluded studies and reasons for their exclusion in the Characteristics of excluded studies tables.
Data extraction and management
Two review authors (GS and JC) independently performed data extraction. One review author (GS) entered final data into Review Manager (RevMan 2014), and a second review author (JC) checked the data. A third review author (RM) was available to resolve any differences through discussion. We attempted to obtain clarification regarding unclear data from the trial authors. The review authors were not blinded to the names of trial authors, journals, or institutions.
Assessment of risk of bias in included studies
We used The Cochrane Collaboration's tool for assessing risk of bias (Higgins 2011). We evaluated the following relevant domains:
sequence generation;
allocation concealment;
blinding of participants, personnel, and outcome assessors;
incomplete data for outcomes (participants lost to follow‐up, intention‐to‐treat analysis (participants analysed in the groups to which they were randomised), and if differences between comparison groups were found);
selective reporting of outcomes; and
biases from other sources.
Specific methodological assessment included the following: aims clearly defined, information about interventions (drug doses and treatment duration) and outcome measures clearly stated; whether participants were assessed for baseline balance in terms of age, sex, duration of psoriasis, and the severity of the disease; whether the statistical analyses used were appropriate for the types of variables.
Measures of treatment effect
We defined successful treatment as the proportion of participants obtaining a PASI 75. If this was not available, we used PASI 50, PASI 90, or a PGA of "almost clear" or better.
For dichotomous variables, we expressed the results as risk ratios and 95% confidence intervals (CI). For continuous variables, we planned to use the mean difference with its 95% CI. We also expressed the results as number needed to treat for an additional beneficial outcome, where appropriate.
Unit of analysis issues
We planned to include only parallel design trials, thus the unit of analysis was the child of each trial.
Dealing with missing data
We contacted trial authors in order to obtain missing data. If we were unable to obtain missing data, we planned to conduct sensitivity analyses, imputing missing data considering the best‐ and the worst‐case scenario.
Assessment of heterogeneity
We planned to evaluate clinical, statistical, and methodological heterogeneity. However, we were unable to do this since we identified only one eligible study.
If future updates of this review include new studies, we will assess heterogeneity using the I² statistic (Higgins 2011). If substantial heterogeneity (I² greater than 50%) is found for the primary outcomes, we will explore reasons for heterogeneity, such as disease severity, dosage, and duration of treatment. Where it is not possible to perform a meta‐analysis, we will summarise the data for each trial qualitatively.
Assessment of reporting biases
We planned to explore funnel plot asymmetry following the approach of Egger if there had been a sufficient numbers of included studies (at least 10) (Egger 1997).
If future updates of this review include new studies and at least 10 studies are added, we will assess reporting biases using the funnel plot method and perform tests to evaluate its asymmetry as required.
Data synthesis
We planned to calculate a pooled intervention effect estimate as a weighted average of the intervention effects estimated in the individual studies. We planned to use risk ratios with a random‐effects model for the pooling of dichotomous outcomes, and standardised mean differences with 95% CI to pool continuous outcomes where different scales or cut‐offs have been used.
Subgroup analysis and investigation of heterogeneity
If statistical heterogeneity was present and there were an adequate number of studies, we planned to investigate the potential influence of some variables by conducting subgroup analyses with respect to:
sex;
disease duration;
psoriasis severity; and
body mass index.
In the future, if there are a sufficient number of included studies we will perform subgroup analysis taking into account these variables.
Sensitivity analysis
If there were an adequate number of studies, we would have performed sensitivity analyses based on separation of studies according to the risk of bias of allocation concealment (high, low, or unclear) and blinding of outcome assessment (high, low, or unclear) (Higgins 2011). However, the number of included studies was inadequate to perform sensitivity analyses.
If future updates of this review include new studies, we will assess reporting biases, as planned.
Results
Description of studies
See Characteristics of included studies and Characteristics of excluded studies.
Results of the search
We retrieved 2369 records from our database searches, and were left with 2205 following removal of duplicates. We screened the titles and abstracts of these and excluded 2185 records that did not meet our inclusion criteria. Where possible, we obtained the full text of the remaining 20 records. We excluded two studies that were not RCTs (see Characteristics of excluded studies). Five studies (reported in seven references) were ongoing (see Characteristics of ongoing studies). We included one trial reported in 11 references in this review.
See Figure 1 for a summary of our screening process. After scanning the bibliographies of included studies, we did not find any additional relevant references. We obtained replies from all the pharmaceutical companies we contacted, except from UCB S.A. and Amgen Inc.
1.
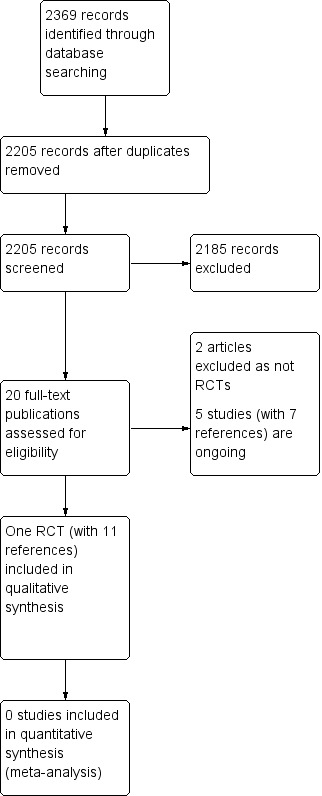
Study flow diagram.
Included studies
For a full description of the included trial see Characteristics of included studies.
We found one study that fulfilled our inclusion criteria with 11 associated publications. Paller 2008 was marked as the primary reference of this group of publications, which assessed the efficacy and safety of etanercept in children and adolescents with moderate to severe plaque psoriasis.
The other publications of this trial are by Langley, which showed the health‐related quality of life (HRQoL) data from the original study (Langley 2011); a post hoc (non a priori) subgroup analysis according to the age of participants of the included study by Landells (Landells 2010); the safety and efficacy results of the final 12‐week, randomised, double‐blind withdrawal and retreatment phase of the included study by Siegfried (Siegfried 2010); the report of a 96‐week open‐label, long‐term extension of the included RCT that was also by Paller (Paller 2010a); another publication by Paller describing the PASI 50 and PASI 75 results by subgroup according to age, gender, BSA, baseline PASI, baseline PGA, disease duration, and previous systemic therapy or phototherapy (Paller 2010b); and five abstracts presented at several meetings regarding different aspects of the included study (Levy 2005; Paller 2007; Paller 2010c; Paller 2010d; Siegfried 2006). According to information provided by Dr. Amy Paller and at the time this review was written, no trials in paediatric psoriasis were ongoing in the United States.
Design
The included study had an initial 12‐week randomised, double‐blind, placebo‐controlled phase (day 1 through to week 12) to assess efficacy; a 24‐week open‐label phase (weeks 13 through to 36) to evaluate the efficacy of etanercept therapy in all participants; and a 12‐week phase of a randomised, double‐blind, withdrawal–retreatment design (weeks 37 through to 48) to evaluate withdrawal effects of the study drug and subsequent retreatment (Paller 2008).
Another associated publication described results after 48 weeks of the former trial in an open‐label design fashion (Paller 2010a).
Participants
The study included 211 participants (106 in the treatment arm, 105 in the placebo arm) with paediatric psoriasis from 4 to 17 years of age, who must have had stable moderate to severe plaque psoriasis at screening, which is defined as a PASI score of at least 12; a static PGA of at least 3 (where 0 indicates clear and 5 indicates severe psoriasis); BSA‐psoriasis involvement of at least 10%; and a history of psoriasis in the last 6 months. They must also have had a poor response or a contraindication to previous or current treatment with phototherapy or systemic psoriasis therapy (for example, retinoids, methotrexate, or ciclosporin) or poorly controlled psoriasis with topical therapy.
The median age of enrolled participants was 13 years, and 64% of participants were older than 11 years of age.
The exclusion criteria of this study were lactation or pregnancy; previous treatment with anti‐TNF agents; guttate, erythrodermic, or pustular psoriasis; major concurrent medical conditions; other skin conditions that would interfere with study evaluations; systemic psoriasis medications; treatment with psoralen and ultraviolet A (PUVA), ultraviolet A, ultraviolet B; oral, parenteral, or topical corticosteroids, D analogue preparations or topical vitamin A, calcineurin inhibitor or anthralin within a 14‐day washout period before the study; and use of any biologic agent within a 30‐day washout period before the study. Participants were allowed to use low to moderate‐potency topical steroids on intertriginous areas and in the scalp.
Setting
Participants were recruited at 42 sites located in the United States and Canada.
Interventions
Participants in the treatment arm received once‐weekly subcutaneous injection of etanercept at a dose of 0.8 mg per kilogram of body weight (maximum dose: 50 mg), whereas participants in the placebo arm received matching placebo. Follow‐up was initially over a 48‐week period in the original RCT (Paller 2008), but the active intervention was provided in an open‐label fashion later on (Paller 2010a).
Outcomes
The primary outcome of the sole included study was PASI 75 at week 12. Secondary efficacy endpoints were PASI 90, PASI 50, a PGA of clear or almost clear (score of 0 or 1), and CDLQI at week 12, which were evaluated at weeks 2, 4, 8, and 16 and every 4 weeks thereafter, as well as the improvement of the mean percentage in PASI score at all time points.
Participants were randomised to etanercept or placebo for 12 weeks. Thereafter at week 13 all participants received etanercept in an open‐label scheme until week 36. At week 37, participants who achieved 75% improvement in PASI from baseline (PASI 75) were re‐randomised for a double‐blind withdrawal and retreatment period for another 12 weeks. During this phase, participants received either placebo or etanercept as long as they maintained a clinical response, defined as PASI 75. Participants whose response fell below PASI 75 were retreated with etanercept in an open‐label fashion until study completion. PASI 75 was assessed every four weeks during the withdrawal and retreatment period.
Patient‐reported outcomes in the Langley publication included the CDLQI, Pediatric Quality of Life Inventory (PedsQL), Stein Impact on Family Scale, and Harter Self‐Perception Profile for Children. The CDLQI was administered at baseline and at weeks 2, 4, and 12 during the double‐blind period. The other three scales were completed at baseline and at week 12 (Langley 2011).
Safety outcomes included non‐serious adverse events, serious adverse events, as well as non‐serious infections, serious infections, malignancies, injection‐site reactions, laboratory results, etanercept concentration in serum, and disease recurrence during the withdrawal period, which was defined as "the worsening of PASI by more than 125% from baseline within 3 months after discontinuation of treatment".
In the 96‐week open‐label trial extension, the primary endpoint was the occurrence of adverse events and secondary efficacy endpoints included PASI 50, ‐75,‐90, CDLQI, improvement in joint pain, and static Physician's Global Assessment (Paller 2010a).
Excluded studies
We excluded two studies: One was a report of paediatric psoriasis cases treated with etanercept that was not a RCT (Beikert 2012), and the other was a retrospective study (Alsuwaidan 2011). (See Figure 1.)
Ongoing studies
We identified five ongoing trials by searching trials registers (see Characteristics of ongoing studies for details). One ongoing study, NCT01251614, has been presented in several preliminary publications, two as meeting abstracts: one, Papp 2014, presented the design of the study and the PGA, CDLQI, and itch visual analogue scale improvements according to baseline characteristics of the participants, and the other, Papp 2015, included efficacy and safety results presented as a poster during the last World Dermatology Congress held in Vancouver, Canada in June 2015. Once this ongoing trial is published, we will consider it for inclusion in future updates of this review.
Risk of bias in included studies
Please see Figure 2, which shows our judgement of the risk of bias for the following domains.
2.
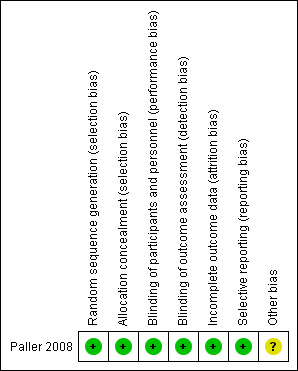
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
We judged the original published study to be at low risk of bias for random sequence generation, as the participants underwent randomisation in a 1:1 ratio by an interactive voice‐response system (Paller 2008). According to information provided by Pfizer, Amgen generated the allocation sequence, so the identity of the investigational product assigned to participants was concealed using an interactive voice‐response system.
Blinding
The study was described as “double‐blind” in the abstract and methods, but it was not clear who was blinded and whether blinded outcome assessment was attempted. We therefore contacted the main author and Pfizer, who confirmed that all participants, study site personnel, and Amgen staff were blinded until the data up to week 12 was finalised. They confirmed that the outcome evaluators were dermatologists or dermatologists in training, who were certified in the use of PASI training materials and blinded as outcome assessors.
Incomplete outcome data
In the original study, analysis was performed according to the intention‐to‐treat principle (ITT) during the first 12 weeks, thus bias was avoided. However, analysis of the 12‐week randomised, double‐blind withdrawal and retreatment period (over the weeks 37 to 48) was not performed according to the ITT principle.
We contacted one of the trial authors to try to obtain trial‐level data, which was not originally reported. Although we received a reply, the trial author was not able to provide any further data and referred us to the pharmaceutical company, who was the sponsor of the only included study (Amgen‐Immunex). We finally obtained a reply from Pfizer (which comarkets etanercept with Amgen in the United States), but they replied that only the data described in the primary publication was available, as follows: "A non response imputation was applied to post‐baseline data that were missing and to all efficacy endpoints after patients entered the escape group; missing data of binary endpoints were imputed as non‐responses, and missing data of continuous endpoints were imputed to include all baseline values".
Selective reporting
There was no evidence of selective reporting in this single included study.
Other potential sources of bias
Participants had stable moderate to severe plaque psoriasis at screening (defined as a PASI score equal to or greater than 12); stable disease; PGA of at least 3; BSA‐psoriasis involvement of at least 10%; a history of psoriasis in the last 6 months; and previous or current treatment with phototherapy or systemic psoriasis therapy (for example retinoids, MTX, or ciclosporin) or poorly controlled psoriasis with the use of topical therapy.
The control arm had slightly lower disease duration at 5.8 years compared to 6.8 years in the intervention group. More participants had a history of previous systemic therapy or phototherapy in the control group at 59%, versus 55% in the intervention group, and there was more psoriatic arthritis in the control group (13% versus 5%) at baseline. Although it is unlikely that these differences between the groups were clinically relevant, it remains unclear if the higher percentage of participants with psoriatic arthritis in the placebo group had an impact on CDLQI scores at week 12. Participant demographics, PASI score, affected percentage of BSA, and PGA prior to entry into the study did not differ between the two groups.
This was the only RCT found, and it was an industry‐sponsored trial. We therefore sought out missing or unclear information initially by contacting the principal investigator and thereafter by contacting the pharmaceutical laboratory (Pfizer), which provided clearer information in April and May 2013 regarding sequence allocation, allocation concealment, blinding of participants and personnel, and blinding of outcome assessment.
Effects of interventions
See: Table 1
The only comparison was between etanercept and placebo. Our prespecified timings were: less than three months, between three months and one year, and after one year. We grouped these into short, medium, and long term, according to how they were assessed, so these fit well with the main and secondary endpoint data in the original included study, which were evaluated at 12 (short term), and at 24, 36, and 48 weeks (medium term). Long‐term data was reported in one of the publications associated with the primary study (Paller 2010a), in which the authors describe an open‐label extension phase of 96 weeks, after the former 48 weeks of the original study.
We expressed efficacy results as risk ratios (RR) with their respective 95% CI, and for such calculations, events obtained in all scales were labelled as improvement. For CDLQI, the percentage change towards improvement at week 12 was presented, as reported in the original study (Paller 2008). For this outcome, we also calculated the mean difference (MD) between groups according to data presented in another publication of the included study (Langley 2011). No meta‐analyses were possible, but we have presented the results for primary endpoints using forest plots.
As several trials of anti‐TNF agents in paediatric psoriasis are ongoing, in future updates of this review we plan to perform meta‐analyses if there are sufficient numbers of included studies.
Primary outcomes
1) Investigator‐assessed improvement: proportion of participants achieving PASI 75
PASI corresponds to the Psoriasis Area and Severity Index. PASI 50 and PASI 75 mean improvements in the PASI of 50% and 75%, respectively, when compared to baseline.
At week 12 (short term), 60 out of 106 participants (57%) who received etanercept achieved PASI 75 compared to 12 out of 105 (11%) who received placebo (RR 4.95, 95% CI 2.83 to 8.65; high‐quality evidence) (Analysis 1.1, Figure 3). The reduction of the absolute risk and the number needed to treat to obtain a benefit with etanercept was 45% (95% CI 33.95 to 56.40) and 2 (95% CI 1.77 to 2.95), respectively. In subgroup analysis and at week 12, 38 out of 59 participants (64%) of the lower‐dose (0.8 mg/kg) group (37 children and 22 adolescents) achieved PASI 75, as compared with 22 out of 47 participants (47%) receiving the higher dose of etanercept (maximum 50 mg) (1 child and 46 adolescents). The response rate of PASI 75 was 58% in children and 56% in adolescents in the etanercept group at week 12 (short term) (Paller 2008).
1.1. Analysis.
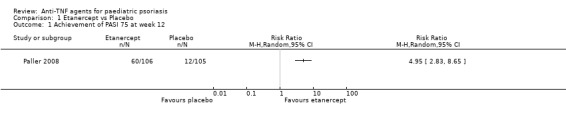
Comparison 1 Etanercept vs Placebo, Outcome 1 Achievement of PASI 75 at week 12.
3.

Forest plot of comparison: 1 Etanercept vs Placebo, outcome: 1.1 Achievement of PASI 75 at week 12.
During the open‐label period (week 12 through to week 24) (medium term), all participants received etanercept without blinding. Among these, 64 out of 103 participants (62%) in the original placebo group and 72 out of 105 participants (69%) in the original etanercept group achieved PASI 75, which was maintained to week 36.
At week 36, "94% of individuals in each treatment group began this phase with a PASI 75 response" (Paller 2008). From this point, 138 out of 211 participants started a withdrawal–treatment period (week 36 through week 48), in which participants were randomly assigned either to continue etanercept or to switch to placebo. After this period and at week 48, 29 out of 69 participants (42%) assigned to placebo at week 36 lost their PASI response (and were switched to etanercept) compared with 19% assigned to etanercept (Siegfried 2010).
After week 48, an extension study period was added, in which only 126 out of 211 participants remained in the study at 96 weeks (Paller 2010a). Sixty‐one percent of participants in this extension study achieved PASI 75 at week 96 (Paller 2010a). In this phase of the study, sensitivity analysis was performed, showing a PASI 75 in 58% of participants using the last observation carried forward (LOCF) imputation method, and 46% using imputation according to treatment failure.
2) Improvement in quality of life
According to the HRQoL published study (Langley 2011), the CDLQI was assessed at baseline and at weeks 2, 4, and 12 during the double‐blind period. The cartoon version of the CDLQI was completed by children 4 to 12 years of age. Adolescents 13 to 17 years of age completed the written version. In the included study at week 12 participants in the etanercept group demonstrated greater improvement from baseline in total CDLQI scores when compared to the placebo group (52.3% (median 66.7%) versus 17.5% (median 15.5%), respectively (P = 0.0001) (Langley 2011). Indeed, participants with PASI 75 at week 12 who were treated with etanercept had CDLQI total score improvements at weeks 2, 4, and 12 compared to participants treated with etanercept who did not achieve PASI 75 (Langley 2011). At week 36, the mean improvements in CDLQI were 63% and 59% for the original etanercept group and the placebo group, respectively.(Langley 2011).
The CDLQI total scores at baseline and weeks 24, 48, 72, and 96 were 59.5%, 55.0%, 59.2%, 57.5%, and 61.1%, respectively compared to an improvement of mean percentages of 59.5% at baseline, 55% at 24 weeks, 57.3% at 48 weeks, 51.7% at 72 weeks, and 54.9% at 96 weeks using the imputation method of the LOCF, whereas using treatment failure imputation mean percentages were 59.5%, 49.3%, 50.7%, 45.2%, and 44.5%, respectively (Paller 2010a).
Our assessment of the MD between groups according to the data described in the Langley 2011 publication showed an improvement in quality‐of‐life scores in participants treated with etanercept (Figure 4; Analysis 1.2), as the CDLQI minimally important difference (MID) was established to be 2.5 (Langley 2011; Lewis‐Jones 1995). Although the MID was also reached in the placebo group, the effect size was clinically important (MD 2.30, 95% CI 0.85 to 3.75; moderate quality evidence). Also, as shown in Langley 2011, when median values were compared, only etanercept‐treated participants achieved the MID. However, such analysis of the means, medians, and MID and the following participant‐reported outcomes endpoints were only exploratory, as they were not specified a priori.
4.

Forest plot of comparison: 1 Etanercept vs Placebo, outcome: 1.2 Children's Dermatology Life Quality Index (CDLQI) response. (Data were extracted from Langley 2011. Means calculation for each group was not a prespecified outcome.)
1.2. Analysis.
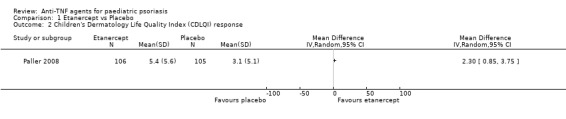
Comparison 1 Etanercept vs Placebo, Outcome 2 Children's Dermatology Life Quality Index (CDLQI) response.
The PedsQL was also assessed at baseline and at week 12 during the double‐blind period. Children 4 to 7 years of age had caregiver or parent assistance for both tests. Mean total PedsQL scores were similar in both the etanercept and placebo groups at baseline (74.8 and 76.1, respectively), but at week 12 significant improvement was not found, although slightly higher mean PedsQL total scores were found for the etanercept group versus the placebo group (81.7 and 79.8, respectively).
The Stein Impact on Family Scale assessed the impact of psoriasis on the lives of participants’ families. During the 12‐week double‐blind phase, caregivers or parents completed the questionnaire, obtaining similar scores at baseline (etanercept‐group mean total score: 46.3; placebo‐group mean total score: 46.1) compared to week 12 (etanercept‐group mean total score: 48.2; placebo‐group mean total score: 47.4).
The Harter Self‐Perception Profile for Children (with two versions according to participant's age) evaluated the impact of psoriasis on participants’ self esteem. No improvement was found in this scale over time (Langley 2011).
3) Proportion of participants having any minor or major adverse outcomes
In the first published study (Paller 2008), the proportion of participants having adverse events was not specified. Adverse outcomes were depicted as "exposure‐adjusted rates for which there were at least 10 events per 100 patient‐years in the etanercept group" (Paller 2008). Also, as described in the article, "the number of exposure‐years was 18.8 for the placebo group and 164.8 for the etanercept group" (Paller 2008).
The rates of non‐infectious adverse events corresponded to "430.5 per 100 patient‐years for placebo and 287.6 per 100 patient‐years for etanercept" (Paller 2008). Infection rates varied from 229.3 per 100 patient‐years in the etanercept group to 308.3 per 100 patient‐years in the placebo group. The majority were not serious, except for 10 events (placebo group: 3; etanercept group: 7). Reactions in the injection site were not serious and generally transient. During the double‐blind phase of the study, the most common events were upper respiratory tract infection, nasopharyngitis, and headache. Overall, no serious adverse events occurred during the placebo‐controlled phase.
During the open‐label phase of treatment, the only information that was provided in the first published study included three serious adverse events that were observed: a 7‐year‐old girl with a history of asthma had a basilar pneumonia requiring hospitalisation, intravenous antibiotics, and etanercept discontinuation; a 9‐year‐old with gastroenteritis and dehydration required hospitalisation; and a 14‐year‐old with a haemorrhagic ovarian cyst required surgical removal and etanercept discontinuation (Paller 2008). No sequelae were reported after these adverse events were resolved. No deaths, demyelination events, malignant tumours, tuberculosis, or opportunistic infections were reported (Paller 2008). Three participants had abnormal haemoglobin concentrations (a grade 3 toxic effect) that self resolved. No grade 4 toxic effects were reported in either group (Paller 2008). During the withdrawal‐retreatment period, no serious adverse events were encountered, and no participant withdrew as a consequence of an adverse event (Siegfried 2010).
In the open‐label phase of the original trial (from week 48 to week 96), adverse events occurred in at least 5% of participants, with 80.1% of participants having one or more adverse events (upper respiratory tract infections in 24.9%, nasopharyngitis in 17.1%, streptococcal pharyngitis in 12.7%, headache in 11.6%, and sinusitis in 10.5%) (Paller 2010a). Through the 96‐week extension trial, two participants withdrew from the study due to an adverse event or infection not related to etanercept (sinusitis and Crohn's disease), and no deaths, opportunistic infections, or malignancies were reported (Paller 2010a).
Secondary outcomes
1) Proportion of participants achieving PASI 50, PASI 90, or both
More participants in the etanercept group versus the placebo group, 79 out of 106 (75%) versus 24 out of 105 (23%), respectively, achieved PASI 50 (RR 3.26, 95% CI 2.26 to 4.71; high‐quality evidence; Analysis 1.3), and PASI 90 occurred in 29 out of 106 participants (27%) in the etanercept group versus 7 out of 105 participants (7%) in the placebo group (RR 4.10, 95% CI 1.88 to 8.95; high‐quality evidence; Analysis 1.4).
1.3. Analysis.
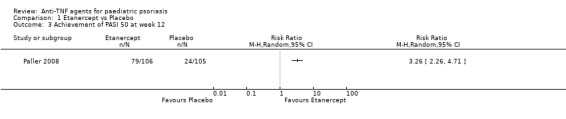
Comparison 1 Etanercept vs Placebo, Outcome 3 Achievement of PASI 50 at week 12.
1.4. Analysis.
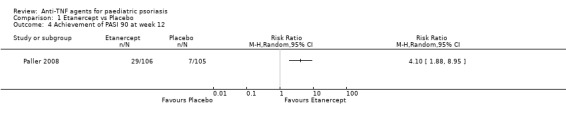
Comparison 1 Etanercept vs Placebo, Outcome 4 Achievement of PASI 90 at week 12.
In the etanercept group at week 12, the response rates of PASI 50 and PASI 90 were 76% and 32%, respectively, in children, and 74% and 25%, respectively, in adolescents.
The study authors included in the analysis the following participants who achieved PASI 75 at week 36: 2 out of 10 participants in the original placebo group and 5 out of 16 participants in the original etanercept group who did not achieve PASI 50 at week 24, and who opted to receive topical standard‐care therapy. There was an increase in the number of participants who achieved PASI 90 and PASI 50 at 24 and 36 weeks (medium term) compared to such results obtained at week 12. Although the study authors did not show if the differences in these measures were statistically significant, according to the article, significant overall improvement was obtained in PASI from baseline when both groups were compared from week 2 (22% in the etanercept group versus 5% in the placebo group, P < 0.001) through to week 12 (68% versus 21%, P < 0.001) (Paller 2008). Likewise, authors reported "71% and 76% of the mean percentage of PASI improvement at weeks 24 and 36, respectively, in the original placebo group, and 77% and 77%, respectively, in the original etanercept group" (Paller 2008). Actual numbers were not reported in the article.
PASI 50 and 90 obtained at week 96 (long term) in the open‐label phase of the primary study were 89% and 30%, respectively (Paller 2010a). In this study, sensitivity analysis was performed, showing PASI 50 and 90 scores of 85% and 29%, respectively, using the LOCF imputation method, and 68% and 23%, respectively, using imputation according to treatment failure (Paller 2010a).
2) Investigator‐assessed improvement: the PGA
Physician’s Global Assessment (PGA) scores ranged from 0 (clear) to 5 (severe psoriasis). A score of equal to or greater than 3 indicated moderate to severe psoriasis. At baseline, 105 out of 106 participants (99%) in the etanercept group and 104 out of 105 participants (99%) in the placebo group had moderate to severe disease, according to the PGA. Fourteen out of 105 participants (13%) in the placebo group and 56 out of 106 participants (53%) in the etanercept group had a PGA of clear or almost clear (RR 3.96, 95% CI 2.36 to 6.66; high‐quality evidence; Analysis 1.5) at week 12, and improvement was seen as early as week 4.
1.5. Analysis.
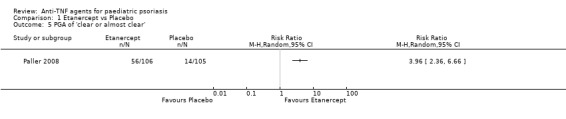
Comparison 1 Etanercept vs Placebo, Outcome 5 PGA of 'clear or almost clear'.
Although the study authors did not show if the differences were statistically significant, 58 out of 103 participants (56%) in the original placebo group had a PGA of clear or almost clear, and in the original etanercept group, the PGA was clear or almost clear in 60 out of 105 participants (57%) at week 24, and in 56 out of 105 participants (53%) at week 36.
During the open‐label phase of the primary study and at 96 weeks (long term), a PGA score of clear or almost clear was 47%, and sensitivity analysis showed a variation from 48% using LOCF imputation to 36% according to treatment failure imputation (Paller 2010a). Joint pain assessment (which was another secondary endpoint included in the 96‐week extension trial) could be evaluated only in 36 participants (20%), therefore analysis of this outcome was inconclusive (Paller 2010a)
3) Investigator‐assessed improvement: affected body surface area
Although the percentage of affected body‐surface area was described at baseline and before the withdrawal–retreatment period (second randomised treatment), no data for statistical analysis was available in the original included study.
4) Participant‐assessed improvement: Patient Global Assessment
We did not assess this outcome.
5) Remission, recurrence, and resource use data
No economic data was available. Participant follow‐up in the original included study lasted just until week 48, so recurrence and remission information after that time was not available.
Discussion
Summary of main results
This systematic review examines and summarises the evidence regarding the efficacy of anti‐TNF agents in paediatric psoriasis. Eleven publications described one RCT (Landells 2010; Langley 2011; Levy 2005; Paller 2007; Paller 2008;Paller 2010a; Paller 2010b; Paller 2010c; Paller 2010d; Siegfried 2006; Siegfried 2010), which compared the use of etanercept versus placebo using the achievement of PASI 75 as the primary outcome. Regarding secondary outcomes, better CDLQI, PASI 50, and PASI 90 scores were found in the etanercept group compared to the placebo group at week 12 (Langley 2011). The risk of bias of the included RCT was low across the majority of the domains assessed, and although we rated publication bias down to the lowest score because we included only one industry‐sponsored study, the GRADE approach still showed high‐quality evidence for all outcomes (except for the assessment of quality of life, which was downgraded to moderate‐quality evidence).
Fifty‐seven percent who received etanercept versus 11% who received placebo achieved the PASI 75 (RR 4.95, 95% CI 2.83 to 8.65; high‐quality evidence). Absolute risk reduction and the number needed to treat to obtain a benefit with etanercept was 45% and 2 (95% CI 1.77 to 2.95), respectively. Also, 13% of participants in the placebo group and 53% in the etanercept group had a PGA of clear or almost clear (RR 3.96, 95% CI 2.36 to 6.66; high‐quality evidence) at week 12. A percentage improvement of CDLQI scores was found in the etanercept group versus the placebo group at week 12 (52.3% versus 17.5%, respectively; P = 0.0001), and analysis showed an effect size that was clinically important (MD 2.30, 95% CI 0.85 to 3.75; high‐quality evidence). However, results of quality‐of‐life scores, other than the percentage of improvement from baseline in the CDLQI through week 12, must be interpreted with caution, as they were not prespecified outcomes.
The most common adverse events during the double‐blind period of the only included RCT were non‐serious. During the open‐label treatment, three serious adverse events were observed, but these were all resolved without sequelae or death. During the withdrawal‐retreatment period, no serious adverse events were reported, and no participants withdrew as a consequence of an adverse event (Siegfried 2010).
Trial authors may depict adverse events as exposure‐adjusted rates. The exposure‐adjusted incidence rate is defined as "the number of subjects with a specific event divided by the total exposure‐time among the subjects in the treatment group and at risk of an initial occurrence of the event" (Liu 2006). However, although such an approach is valid when the specific event‐incidence rate is relatively constant over the duration of the study, it fails to provide adverse effect information of time or exposure duration for all events or for several events that occur in the same individual. In addition, some other adverse events in the included RCT, e.g. upper respiratory tract infection (URTI), might be correlated with nasopharyngitis, and similarly, headache could be correlated with URTI and nasopharyngitis (Siddiqui 2009). Therefore, more advanced statistical methods to evaluate safety are needed. Also, as children could be on lifelong biologic treatment, safety issues must be presented in a friendly format for clinicians.
Although malignancies were not found in the included RCT, an increased risk for the development of malignancies, including lymphoma, has been reported with the use of TNF‐blocking agents (EMA 2011; EMA 2013; US FDA 2009; US FDA 2012). Therefore, there are still uncertainties in this field.
Overall completeness and applicability of evidence
We found only one eligible high‐quality study evaluating anti‐TNF therapy for moderate to severe paediatric psoriasis. The median age of participants in this trial was 13 years (range from 4 to 17 years). Although the applicability of evidence in younger children could be limited, psoriasis in children younger than four years old is unusual and mostly mild and limited to small areas. (Bell 1991; Bronckers 2015) The applicability of the evidence could also be limited due to the fact that only short‐term safety was evaluated and just one type of anti‐TNF agent (etanercept) was used. Due to these limitations, the findings of this review should not be generalised to other types of anti‐TNF agents.
Quality of the evidence
We assessed the quality of evidence for each important outcome through the use of GRADE and rated it as high (Table 1), except for the CDLQI outcome, which we rated as moderate. As only one industry‐sponsored RCT was eligible, risk of bias was strongly suspected, and inconsistency across studies and mid‐ and long‐term safety could not be evaluated. However, this was a double‐blind RCT with adequate blinding of outcome assessors, it had a complete accounting of participants and outcome events, and there was no selective reporting or indirectness.
Potential biases in the review process
According to our rigorous systematic search of published and unpublished literature and since we contacted leading experts, it is unlikely that we have missed studies with substantial numbers of participants. However, the fact that six studies have not yet been incorporated may be a source of potential bias.
As we included only one eligible study, it was not possible to perform a meta‐analysis.
Agreements and disagreements with other studies or reviews
To our knowledge, this is the first systematic review to assess the evidence for the efficacy and safety of anti‐TNF agents for paediatric psoriasis. Current guidelines on the management of psoriasis with systemic therapy have focused mainly on adults (Tan 2010), and there is a paucity of studies of therapies for children with moderate to severe psoriasis. What studies there are have been mainly based on descriptive studies or case series. Therefore, more well‐performed RCTs such as this sole included study are needed as they may provide a body of evidence for the efficacy of systemic treatments for children with moderate to severe psoriasis.
Authors' conclusions
Implications for practice.
Except for the quality‐of‐life outcomes, the quality of evidence of the sole RCT that evaluated the use of this kind of biological therapy was high. This review has concluded that the anti‐TNF agent etanercept is effective in improving psoriasis in a paediatric population as assessed by PASI 75, 50, and 90 and PGA. During the open‐label treatment, three serious adverse events were observed, but all these adverse events were resolved without sequelae or death. Although no serious adverse events were reported in the other phases of the study, mid‐ and long‐term safety were not evaluated.
The GRADE approach refers not to individual studies but to a body of evidence. As this review assessed the quality of evidence of just one individual trial, so far we cannot make a full recommendation for the use of anti‐TNF agents in paediatric psoriasis. We shall therefore wait for the results of the ongoing studies, as the conclusions of this review may be altered once they are available for inclusion in a future update.
Implications for research.
A significant aspect of drug trials is to assess safety alongside efficacy. Well‐designed RCTs assessing the safety (both short and long term) of biological therapies in paediatric psoriasis as one of the main objectives are therefore needed in order to provide high‐quality evidence to ease clinical decision‐making. Furthermore, efficacy and safety trials with a sufficient number of younger participants (less than 11 years old) are also required, as current evidence applies mostly to older children.
Although other RCTs are ongoing, a lack of standardisation of outcomes and time‐frames between studies will impact any meta‐analyses of future evidence in this field. It is therefore necessary to take these factors into account when designing trials in this population in the future. Future studies should preferably consider the following.
PASI 75 and PGA as main outcomes (unless a better and validated measurement tool arises), as well as quality of life and short‐ and long‐term adverse effects.
Given the high cost of these therapies, it would be useful to collect information on cost of treatment.
Standardised outcome measures among RCTs would make studies easier to compare.
More advanced statistical methods should be investigated to report adverse events, and such results need to be presented in a format that is readily understood by clinicians.
We believe that head‐to‐head RCTs evaluating the effectiveness of biologics and classical systemic treatments in paediatric psoriasis would be worthwhile.
What's new
| Date | Event | Description |
|---|---|---|
| 1 May 2019 | Amended | Declarations of interest section updated to clarify the timing of sponsorship of the lead author to confirm that she was not conflicted at the time of publication |
Notes
The Declarations of interest section has been updated to clarify the timing of sponsorship of the lead author to confirm that she was not conflicted at the time of publication, and to add the declarations of three authors as 'none known'.
Acknowledgements
We also wish to thank Jun Xia for her valuable comments and corrections and Dr. Monica Rengifo for her valuable contribution for protocol writing and development. The leading author, Dr. Gloria Sanclemente, is currently a PhD candidate at the Universitat Autònoma de Barcelona (Departamento de Pediatría, de Obstetricia y Ginecología y de Medicina Preventiva), in Barcelona, Spain.
The Cochrane Skin Group editorial base wishes to thank Matthew Grainge, who was the Statistical Editor for this review; Ching‐Chi Chi, who was the Methods Editor; the clinical referees, Irene Lara‐Corrales, Esther Burden‐Teh, and Laurence Le Cleach; and the consumer referee, Carolyn Hughes.
Appendices
Appendix 1. Skin Group Specialised Register search strategy
(Psoria* or “palmoplantar* pustulosis” or “pustulosis palmaris et plantaris” or (pustulosis and palms and soles)) and (“Tumor Necrosis Factor*” or “TNF” or “tumour necrosis factor” or “antitumor necrosis factor” or “antitumour necrosis factor” or “monoclonal antibod*” or “Immunoglobulin Fab Fragments” or infliximab* or “cA2” or remicade or “cdp571” or etanercept* or enbrel or adalimumab* or “d2e7” or humira or golimumab or simponi or Briakinumab or “ABT‐874”)
Appendix 2. CENTRAL (Cochrane Library) search strategy
#1 MeSH descriptor Psoriasis explode all trees #2 (psoria* ) or (palmoplantar* pustulosis) or (pustulosis palmaris et plantaris) or (pustulosis and palms and soles) #3 (#1 OR #2) #4 MeSH descriptor Tumor Necrosis Factor‐alpha, this term only #5 MeSH descriptor Receptors, Tumor Necrosis Factor, this term only #6 MeSH descriptor Receptors, TNF‐Related Apoptosis‐Inducing Ligand, this term only #7 (tumour necrosis factor*) or (tumor necrosis factor*) or (antitumour necrosis factor) or (antitumor necrosis factor) or "tnf" #8 (tnf antibod*) or (tnf alpha antibod*) or (monoclonal antibod*) #9 MeSH descriptor Antibodies, Monoclonal, this term only #10 MeSH descriptor Immunoglobulin Fab Fragments, this term only #11 MeSH descriptor Tumor Necrosis Factors, this term only #12 MeSH descriptor Receptors, Tumor Necrosis Factor, Type II, this term only #13 MeSH descriptor Receptors, Tumor Necrosis Factor, Type I, this term only #14 (infliximab ) or (monoclonal antibody cA2) or (remicade) or "cdp571" #15 (etanercept* or enbrel) or (adalimumab* or "d2e7" or humira) or (golimumab or simponi) or (briakinumab or "abt‐874") #16 (#4 OR #5 OR #6 OR #7 OR #8 OR #9 OR #10 OR #11 OR #12 OR #13 OR #14 OR #15) #17 (#3 AND #16)
Appendix 3. MEDLINE (OVID) search strategy
1. exp Psoriasis/ or psoria$.mp. 2. palmoplantar$ pustulosis.mp. 3. pustulosis palmaris et plantaris.mp. 4. (pustulosis and palms and soles).mp. 5. or/1‐4 6. exp Tumor Necrosis Factors/ or exp Tumor Necrosis Factor‐alpha/ or exp Receptors, Tumor Necrosis Factor, Type II/ or exp Receptors, Tumor Necrosis Factor/ or exp Receptors, Tumor Necrosis Factor, Type I/ or exp TNF‐Related Apoptosis‐Inducing Ligand/ 7. (anti tumour necrosis factor or anti tumor necrosis factor).mp. 8. (tumor necrosis factor‐alpha or tumour necrosis factor‐alpha).mp. 9. anti tnf.mp. 10. (tnf antibod$ or tnf alpha antibod$).mp. 11. (tumour necrosis factor antibod$ or tumor necrosis factor antibod$).mp. 12. (antitumor necrosis factor or antitumour necrosis factor).mp. 13. exp Antibodies, Monoclonal/ or monoclonal antibod$.mp. 14. exp Immunoglobulin Fab Fragments/ 15. (infliximab$ or monoclonal antibody cA2 or remicade).mp. 16. cdp571.mp. 17. (etanercept$ or enbrel).mp. 18. (adalimumab$ or d2e7 or humira).mp. 19. (golimumab or simponi).mp. 20. (Briakinumab or ABT‐874).mp. 21. or/6‐20 22. adolescent.tw. 23. children.tw. 24. Child, Preschool/ 25. 22 or 23 or 24 26. randomised controlled trial.pt. 27. controlled clinical trial.pt. 28. randomized.ab. 29. placebo.ab. 30. clinical trials as topic.sh. 31. randomly.ab. 32. trial.ti. 33. 26 or 27 or 28 or 29 or 30 or 31 or 32 34. (animals not (humans and animals)).sh. 35. 33 not 34 36. 5 and 21 and 25 and 35
Lines 22‐25 are an age filter from the HEDGES team ‐ see Kastner M, Wilczynski NL, Walker‐Dilks C, McKibbon KA, Haynes B. Age‐specific search strategies for Medline. Journal of Medical Internet Research 2006;8(4):e25.
Lines 26‐35: Cochrane Highly Sensitive Search Strategy for identifying randomized trials in MEDLINE: sensitivity‐ and precision‐maximizing version (2008 revision).
Appendix 4. Embase (OVID) search strategy
1. exp *PSORIASIS/ 2. psoria$.ti,ab. 3. palmoplantar$ pustulosis.ti,ab. 4. pustulosis palmaris et plantaris.ti,ab. 5. (pustulosis and palms and soles).ti,ab. 6. 1 or 2 or 3 or 4 or 5 7. exp *tumor necrosis factor/ or exp *tumor necrosis factor alpha/ 8. exp *tumor necrosis factor receptor 1/ 9. exp *tumor necrosis factor receptor 2/ or exp *tumor necrosis factor receptor/ 10. exp *tumor necrosis factor related apoptosis inducing ligand/ 11. anti tumour necrosis factor.ti,ab. 12. anti tumor necrosis factor.ti,ab. 13. tumour necrosis factor alpha.ti,ab. 14. tumor necrosis factor alpha.ti,ab. 15. anti tnf.ti,ab. 16. tnf inhibitor$.ti,ab. 17. tnf antibod$.ti,ab. 18. tnf alpha antibod$.ti,ab. 19. tumour necrosis factor antibod$.ti,ab. 20. tumor necrosis factor antibod$.ti,ab. 21. antitumor necrosis factor.ti,ab. 22. antitumour necrosis factor.ti,ab. 23. exp *monoclonal antibody/ 24. monoclonal antibod$.ti,ab. 25. exp *"immunoglobulin F(ab) fragment"/ 26. exp *infliximab/ 27. infliximab$.ti,ab. 28. monoclonal antibody cA2.ti,ab. 29. remicade.ti,ab. 30. cdp571.ti,ab. 31. exp *etanercept/ 32. (etanercept$ or enbrel).ti,ab. 33. exp *adalimumab/ 34. (adalimumab$ or humira or "d2e7").ti,ab. 35. exp *golimumab/ 36. (golimumab$ or simponi).ti,ab. 37. exp *briakinumab/ 38. (briakinumab$ or "abt‐874").ti,ab. 39. or/7‐38 40. random$.mp. 41. factorial$.mp. 42. (crossover$ or cross‐over$).mp. 43. placebo$.mp. or PLACEBO/ 44. (doubl$ adj blind$).mp. 45. (singl$ adj blind$).mp. 46. (assign$ or allocat$).mp. 47. volunteer$.mp. or VOLUNTEER/ 48. Crossover Procedure/ 49. Double Blind Procedure/ 50. Randomized Controlled Trial/ 51. Single Blind Procedure/ 52. 40 or 41 or 42 or 43 or 44 or 45 or 46 or 47 or 48 or 49 or 50 or 51 53. 6 and 39 and 52
Appendix 5. LILACS search strategy
We applied two strategies for searching in LILACS. The first one included the terms: ("pustulosis palmoplantar" or psoria$ or "palmoplantar pustulosis" or "pustulosis palmaris et plantaris" or (pustulosis and palms and soles)) and ("tumor Necrosis Factor" or "TNF" or "antitumor necrosis factor" or "antitumour necrosis factor" or "monoclonal antibodies" or "Immunoglobulin Fab Fragments" or infliximab$ or "cA2" or remicade or "cdp571" or etanercept$ or enbrel or adalimumab$ or "d2e7" or humira or golimumab or simponi or Briakinumab or "ABT‐874"). This strategy combined with the Controlled clinical trials topic‐specific query filter.
The second strategy included the terms: Tw estud$ OR Tw Clin$ OR AB grupo$ OR CT COMPARATIVE STUDY OR Tw placebo$ OR Tw randomS Ti compara$ OR Ti tratamiento OR Tw control$ OR MH / dt
Appendix 6. Clinical trials registers' search strategy
This search included the following individual terms in each database: "psoriasis", "children" "pediatric" "paediatric".
Appendix 7. EMA search strategy
This search included the following individual terms: "Etanercept", "Infliximab", and "Adalimumab".
Data and analyses
Comparison 1. Etanercept vs Placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Achievement of PASI 75 at week 12 | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 2 Children's Dermatology Life Quality Index (CDLQI) response | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 3 Achievement of PASI 50 at week 12 | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 4 Achievement of PASI 90 at week 12 | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 5 PGA of 'clear or almost clear' | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Paller 2008.
| Methods | Study design had a 12‐week randomised, double‐blind, placebo‐controlled period, thereafter a 24‐week open‐label phase, and lastly a randomised, double‐blind withdrawal–retreatment period from week 37 to 48 | |
| Participants | 211 participants (106 in the treatment arm, 105 in the placebo arm) with paediatric psoriasis from 4 to 17 years of age were recruited at 42 sites in the United States and Canada Inclusion criteria: Moderate to severe plaque psoriasis at screening (defined as a PASI score of at least 12); stable disease; PGA of at least 3; BSA‐psoriasis involvement of at least 10%; history of psoriasis in the last 6 months; and previous or current treatment with phototherapy/systemic psoriasis therapy (e.g. retinoids, methotrexate, or ciclosporin) or poorly controlled psoriasis with the use of topical therapy |
|
| Interventions | Participants in the treatment arm received a dose of 0.8 mg per kilogram of body weight up to a maximum intended dose of 50 mg of reconstituted etanercept in syringes for once‐weekly subcutaneous injections; participants in the placebo arm received matching placebo | |
| Outcomes | The primary outcome was PASI 75 (improvement in the PASI of 75%) at week 12. Secondary efficacy endpoints were PASI 50 (improvement in the PASI of 50%), PASI 90 (improvement in the PASI of 90%), and a PGA of clear or almost clear (score of 0 or 1), which were evaluated at weeks 2, 4, 8, and 16 and every 4 weeks thereafter The following participant‐reported outcomes were assessed during the double‐blind period in this study:
Safety outcomes included non‐serious adverse events, serious adverse events, non‐serious infections, serious infections, malignancies, reactions in the injection site, laboratory findings, etanercept concentration in serum, and disease recurrence during the withdrawal period, which was defined as "the worsening of PASI by more than 125% from baseline within 3 months after discontinuation of treatment" (Paller 2008) |
|
| Notes | The analysis of the study was designed by Amgen Inc and funded by Immunex Corp, a wholly owned subsidiary of Amgen Inc, and by Wyeth, which was acquired by Pfizer Inc in October 2009. Financial support for the preparation of the manuscript was provided by Amgen Inc | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | In the included study participants underwent randomisation at a 1:1 ratio by an interactive voice‐response system |
| Allocation concealment (selection bias) | Low risk | Not specifically stated in paper, therefore we contacted the trial authors for further details about allocation concealment; since the main investigator was not able to provide further data, we contacted Pfizer. According to information provided by Pfizer, the allocation sequence was generated by Amgen and provided by the interactive voice‐response system by an unblinded randomisation group within Amgen According to the pharmaceutical lab response, the identity of the investigational product assigned to participants was concealed using an "interactive voice response system" |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Stated as "double‐blind" in abstract and methods of the original study. We therefore contacted the main author and Pfizer, who confirmed that all participants, study site personnel, and Amgen staff were blinded until the data through week 12 were finalised |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | It was not clear whether blinded outcome assessment was attempted in the original study. We therefore contacted the main author and Pfizer, who confirmed that outcome evaluators were dermatologists or dermatologists in training who were certified on PASI training materials and were also blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | In the included study, analysis was performed according to the intention‐to‐treat principle during first 12 weeks, thus, avoiding bias, at least in this phase of the study |
| Selective reporting (reporting bias) | Low risk | There was no evidence of selective reporting in the included study |
| Other bias | Unclear risk | Paediatric participants (aged 4 to 17 years) had stable moderate to severe plaque psoriasis at screening (defined as a PASI score >= 12); stable disease; PGA of at least 3; BSA‐psoriasis involvement of at least 10%; a history of psoriasis in the last 6 months; and previous or current treatment with phototherapy/systemic psoriasis therapy (e.g. retinoids, methotrexate, or ciclosporin) or poorly controlled psoriasis with the use of topical therapy When compared to the intervention arm, the control group had slightly lower disease duration at 5.8 years versus 6.8 years, slightly more participants with history of previous systemic therapy or phototherapy (59% vs 55% in the intervention group) and more psoriatic arthritis (13% vs 5%) at baseline. In addition, even though participants were required to have a PASI ≥ 12 at baseline, a PGA ≥ 3, and a BSA ≥ 10%, the median baseline PASI was 16. Also, an important percentage of participants in both arms (45% in the etanercept group and 41% in the placebo group) had no previous systemic or phototherapy history This was a industry‐sponsored trial with positive results. We therefore sought out missing or unclear information by contacting the pharmaceutical laboratory (Pfizer). They provided clearer information in April and May 2013 regarding sequence allocation, allocation concealment, blinding of participants and personnel, and blinding of outcome assessment |
BSA: body surface area. CDLQI: Children's Dermatology Life Quality Index. PASI: Psoriasis Area and Severity Index. PedsQL: Pediatric Quality of Life Inventory. PGA: Physician's Global Assessment.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Alsuwaidan 2011 | This study is not a randomised controlled trial |
| Beikert 2012 | This study was a German language report of paediatric psoriasis cases treated with etanercept, not a randomised controlled trial |
Characteristics of ongoing studies [ordered by study ID]
NCT01090427.
| Trial name or title | A study of the safety and efficacy of ustekinumab in adolescent patients with psoriasis (CADMUS) |
| Methods | This is a phase 3 multicentre, randomised, double‐blind, placebo‐controlled study evaluating the of efficacy and safety of ustekinumab in the treatment of adolescent participants with moderate to severe plaque‐type psoriasis (CADMUS) |
| Participants | People from 12 to 18 years of age with a diagnosis of plaque‐type psoriasis with or without psoriatic arthritis for at least 6 months and who are candidates for phototherapy or systemic treatment of psoriasis and who have screening laboratory test results within the study parameters. Exclusion criteria are: people with non‐plaque forms of psoriasis or who have used any therapeutic agent targeted at reducing interleukin‐12 or interleukin‐23, including but not limited to ustekinumab and briakinumab; who received conventional systemic therapies or phototherapy within the last 4 weeks or who received biologic therapies within the last 3 months |
| Interventions | Ustekinumab half‐standard dosage (ustekinumab 0.375 mg/kg, 22.5 mg, or 45 mg based on body weight, administered subcutaneously (under the skin) at weeks 0, 4, 16, 28, and 40). In addition, all participants will receive a single subcutaneous dose of placebo at week 12 Ustekinumab standard dosage (ustekinumab 0.75 mg/kg, 45 mg, or 90 mg based on body weight administered subcutaneously at weeks 0, 4, 16, 28, and 40). In addition, all participants will receive a single subcutaneous dose of placebo at week 12 Placebo administered subcutaneously at weeks 0 and 4 or at week 12 |
| Outcomes | Primary outcome: proportion of participants who achieve a PGA score of cleared or minimal disease at week 12 Secondary outcome measures: proportion of participants who achieve a PASI 90 response, proportion of participants who achieve PASI 75 response, and the change from baseline in CDLQI at week 12 |
| Starting date | May 2010 |
| Contact information | Study director: Janssen Research & Development, LLC Clinical Trial. Janssen Research & Development, LLC |
| Notes | This study is sponsored by Janssen Research & Development, LLC. The results are posted at www.clinicaltrials.gov (www.clinicaltrials.gov/ct2/show/results/NCT01090427). Last access date: 9 July 2015 |
NCT01100034.
| Trial name or title | Long‐term, prospective, observational cohort study of safety and effectiveness of pediatric psoriasis patients treated with etanercept in a naturalistic setting: A Post‐Authorization Safety Study (PASS) |
| Methods | Phase 4 cohort, prospective, observational cohort study to assess safety and effectiveness of etanercept for the treatment of paediatric psoriasis |
| Participants | People from 4 to 17 years of age diagnosed with plaque psoriasis by a dermatologist. Prior to enrolment, there must be a clinical decision to initiate etanercept for the treatment of plaque psoriasis, and etanercept must then be initiated. Included participants must be being actively treated with etanercept, regardless of length of treatment prior to enrolment. Exclusion criteria: Prior therapy with etanercept or other biologic agent and a history of malignancy |
| Interventions | Etanercept |
| Outcomes | Primary outcome measures: number of serious adverse events including serious infections and malignancy during a 5‐year follow‐up, with follow‐up every 3 months for the first 2 years and 6‐monthly for the next 3 years Secondary outcome measures: effectiveness or lack of effectiveness after a 24‐week treatment course |
| Starting date | November 2010 |
| Contact information | Contact: Pfizer's phonecall centre: 1‐800‐718‐1021 |
| Notes | This study is sponsored by Pfizer. Most of the centres are in the recruiting phase. Estimated completion date: June 2018. Last access date: 9 July 2015 |
NCT01251614.
| Trial name or title | A double blind study in pediatric subjects with chronic plaque psoriasis, studying adalimumab vs. methotrexate |
| Methods | This is a phase III multicentre, randomised, double‐dummy, double‐blind clinical trial, in which 2 doses of adalimumab vs methotrexate will be evaluated in paediatric participants with chronic plaque psoriasis Primary outcomes: PASI 75 at week 16, period A (standard dose vs methotrexate) and the proportion of participants achieving a Physician's Global Assessment of Disease Activity of 0 or 1 at week 16, period A (standard dose vs methotrexate) and adverse events at every visit from baseline (week 0) to final visit (week 156) Secondary outcomes include: The proportion of participants achieving PASI 90, PASI 100; change from baseline in the CDLQI scores; change from baseline in the PedsQL at week 16, period A (standard dose vs methotrexate); the proportion of participants achieving PGA 0, 1 upon completion of retreatment (period C) according to the original randomised group assignment in period A (standard‐dose adalimumab vs low‐dose adalimumab) and time to loss of disease control (period B) according to the original randomised group assignment in period A (standard‐dose adalimumab vs low‐dose adalimumab and methotrexate) |
| Participants | People 4 to 17 years of age with body weight equal to or > 13 kilograms who failed to respond to topical therapy with a PGA equal to or > 4; BSA involved > 20%; people with very thick lesions with BSA > 10%; PASI > 20; PASI > 10 and at least 1 of the following: active psoriatic arthritis unresponsive to nonsteroidal anti‐inflammatory drugs; clinically relevant facial involvement; clinically relevant genital involvement; clinically relevant hand or foot, or both involvement; and CDLQI > 10
If person is < 12 years of age and resides in a geographic region where heliotherapy is practical, person must have failed to respond, be intolerant, or have a contraindication to heliotherapy, or is not a suitable candidate for heliotherapy. If person is equal to or > 12 years of age, person must have failed to respond, be intolerant, or have a contraindication to phototherapy, or is not a suitable candidate for phototherapy. Study participants must have a clinical diagnosis of psoriasis for at least 6 months as determined by the person's medical history and confirmation of diagnosis through physical examination by the investigator and a stable plaque psoriasis for at least 2 months prior to baseline Exclusion criteria: Prior biologic use other than prior treatment with etanercept; treatment with etanercept therapy within 4 weeks prior to the baseline visit; methotrexate use within the past year or prior methotrexate use at any time where the person did not respond or did not tolerate methotrexate; contraindication for treatment with methotrexate during the study; erythrodermic, generalised, or localised pustular psoriasis; medication‐induced or medication‐exacerbated or new‐onset guttate psoriasis. People with infection(s) requiring treatment with intravenous anti‐infectives within 30 days prior to the baseline visit or with oral anti‐infectives within 14 days prior to the baseline visit were excluded as well as people with treatment of psoriasis with topical therapies such as corticosteroids, vitamin D analogues, or retinoids within 7 days prior to the baseline visit Other exclusion criteria are people with treatment of psoriasis with ultraviolet B phototherapy, excessive sun exposure, or the use of tanning beds within 7 days prior to the baseline visit and with treatment of psoriasis with PUVA phototherapy, non‐biologic systemic therapies for the treatment of psoriasis, or systemic therapies known to improve it within 14 days prior to the baseline visit |
| Interventions |
|
| Outcomes | Primary outcomes: PASI 75 week 16, period A: the proportion of participants achieving a PASI 75 response, standard dose vs methotrexate; PGA 0, 1 week 16, period A: the proportion of participants achieving a PGA 0, 1 standard dose vs methotrexate; adverse events at every visit from baseline (week 0) to final visit (week 156); and any untoward medical occurrence Secondary outcomes: PASI 90 week 16, period A: the proportion of participants achieving a PASI 90, standard dose vs methotrexate; PASI 100 week 16, period A: the proportion of participants achieving a PASI 90, standard dose vs methotrexate; CDLQI at week 16, period A: change from baseline in the CDLQI scores, standard dose vs methotrexate; change from baseline in the PedsQL at week 16, period A: change from baseline in the PedsQL, standard dose vs methotrexate; PGA 0,1 at week 16, period A; the proportion of subjects achieving PGA 0, 1 upon completion of retreatment (period C) according to the original randomised group assignment in period A (standard‐dose adalimumab vs low‐dose adalimumab); time to loss of disease control during time from entry into period B until loss of disease control; time to loss of disease control (period B) according to the original randomised group assignment in period A (standard‐dose adalimumab vs low‐dose adalimumab and methotrexate) |
| Starting date | December 2010 |
| Contact information | Susan Williamson, RN, MSN, MBA, PMP tel: 847‐938‐7491; email: susan.williamson@abbott.com |
| Notes | This study is sponsored by AbbVie (prior sponsor, Abbott). Baseline characteristics of participants enrolled in this ongoing study were presented at the last 4th Congress of the Psoriasis International Network in Paris, France (July 2013). Efficacy and safety results were presented in a poster during the last World Dermatology Congress held in Vancouver, Canada (June 2015) |
NCT01432249.
| Trial name or title | Post marketing surveillance to observe safety and efficacy of Enbrel in pediatric patients with psoriasis |
| Methods | This is a phase IV prospective cohort study to observe safety and efficacy of etanercept (Enbrel®) in paediatric patients with psoriasis |
| Participants | Paediatric patients (ages of 8 ˜ 17) with psoriasis Inclusion criteria are children and adolescents aged 8 to 17 years at time of consent with chronic severe psoriasis that is inadequately controlled by, or who are intolerant to other systemic therapies or phototherapies. Exclusion criteria are people with known hypersensitivity to Enbrel® or any component of the product and people with active infections including chronic or localised infections such as tuberculosis |
| Interventions | Enbrel®, which will be decided by treating physicians |
| Outcomes | Primary outcome: Safety measured by discontinuation due to adverse events Secondary outcomes: Proportion of participants achieving a status on the PGA of psoriasis of clear (0), clear/almost clear (0/1), or clear/almost clear/mild (0/1/2) at 12 and 24 weeks; proportion of participants achieving a 50% and 75% improvement from baseline in PASI over 12 and 24 weeks |
| Starting date | July 2012 |
| Contact information | Contact: Pfizer's phonecall centre: 1‐800‐718‐1021 |
| Notes | This study is sponsored by Pfizer. The setting of this study is Korea because it is required in order for Enbrel® to be approved by the Korea Food and Drug Administration. According to ClinicalTrials.gov, the study was withdrawn prior to enrolment. Last access date: 9 July 2015 |
BSA: body surface area. CDLQI: Children's Dermatology Life Quality Index. PASI: Psoriasis Area and Severity Index. PedsQL: Pediatric Quality of Life Inventory. PGA: Physician's Global Assessment. PUVA: psoralen and ultraviolet A radiation.
Differences between protocol and review
There were minor updates to the Background section.
We planned to use PASI 50 or PASI 90 if PASI 75 was not available. However, we included both outcomes in addition to PASI 75,as they added more efficacy data. The protocol planned to assess psoriasis‐affected body surface area, Patient Global Assessment, remissions, recurrences, and resource use data, but such information was not assessed in the included RCT. In addition, we planned to have a short‐, medium‐, and long‐term assessment of outcomes, but the included RCT evaluated outcomes at a maximum of 48 weeks. However, long‐term data and results were evaluated in a further associated publication of the primary study. Lastly, since we included only one eligible study, it was not possible to perform a meta‐analysis.
Contributions of authors
GS was the contact person with the editorial base. GS co‐ordinated contributions from the coauthors and wrote the final draft of the review. GS and JC screened papers against eligibility criteria. GS obtained data on ongoing and unpublished studies. GS and JC appraised the quality of papers. GS, JC, and RM extracted data for the review and sought additional information about papers. GS and JC entered data into RevMan. GS, JC, RM, and XB analysed and interpreted data. GS, JC, RM, and XB worked on the methods sections. GS, JC, and RM drafted the clinical sections of the background and responded to the clinical comments of the referees. GS, JC, and XB responded to the methodology and statistics comments of the referees. HG was the consumer coauthor and checked the review for readability and clarity, as well as ensuring outcomes were relevant to consumers. GS is the guarantor of the update.
Disclaimer
This project was supported by the National Institute for Health Research, via Cochrane Infrastructure funding to the Cochrane Skin Group. The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, NHS, or the Department of Health.
Sources of support
Internal sources
-
Cochrane Skin Group, UK.
Technical support and guidance
External sources
-
Grupo de Investigación Dermatológica (GRID) (Group of Investigative Dermatology (GRID)), Universidad de Antioquia, Medellín, Colombia.
Affiliation of main author. Methodology support
-
The National Institute for Health Research (NIHR), UK.
The NIHR, UK, is the largest single funder of the Cochrane Skin Group
-
Dermabase Foundation, Colombia.
Another affiliation of main author. Logistics and technical support
Declarations of interest
Dr. Gloria Sanclemente was sponsored by Pfizer for attending dermatology meetings and received an honorarium from Pfizer for presenting or speaking about biosimilars in adults; these were more than three years prior to publication of this review. She was sponsored by Janssen‐Cilag Ltd to attend the Cochrane Colloquium 2015 after this review was accepted for publication.
Dr. Ruth Murphy has not been involved in any educational work, travel grants, or lectures with respect to work with children and biological therapies for the treatment of psoriasis. She has been involved with the following work with adults: she has sat on advisory boards for Janssen‐Cilag Ltd, Abbott, Serono, and Wyeth (Pfizer); she has given educational and non‐promotional lectures, which were paid, about the use of biological therapies in psoriasis for Janssen‐Cilag Ltd and Abbott; she has accepted expenses sponsorship in 2010 only to attend meetings at the American Academy of Dermatology, the British Association of Dermatology, and the European Academy of Dermatology and Venereology from Wyeth (Pfizer), Janssen‐Cilag, and Abbott. In addition, Dr. Murphy has taken part in an industry‐sponsored study (TRANSIT). She also received departmental funds in 2007‐8 to train a nurse to take part in the British Association of Dermatologists Biologic Interventions Register from Pfizer.
Javier Contreras: none known Hermenegildo García: none known Xavier Bonfill Cosp: none known
A clinical referee on this review, Dr Esther Burden‐Teh said: "I have received an educational supplement from Leo Pharma and AbbVie to attend the British Association of Dermatologists Annual Meeting and the American Academy of Dermatology Annual Meeting. Leo Pharma do not produce anti‐TNF agents. AbbVie produce the anti‐TNF agent Humira (adalimumab), which is now licensed to treat paediatric psoriasis in Europe." (EMA 2015).
Edited (no change to conclusions)
References
References to studies included in this review
Paller 2008 {published data only}
- Landells I, Paller AS, Pariser D, Kricorian G, Foehl J, Molta C, et al. Efficacy and safety of etanercept in children and adolescents aged > or = 8 years with severe plaque psoriasis. European Journal of Dermatology 2010;20(3):323‐8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Langley RG, Paller AS, Hebert AA, Creamer K, Weng HH, Jahreis A, et al. Patient‐reported outcomes in pediatric patients with psoriasis undergoing etanercept treatment: 12‐week results from a phase III randomized controlled trial. Journal of the American Academy of Dermatology 2011;64(1):64‐70. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Levy ML, Jahreis A, Paller AS, Walker S, Molta C, Freundlich B. Etanercept in children and adolescents with psoriasis (ABP08‐119). Journal of the European Academy of Dermatology & Venereology 2005;19(Suppl 2):251. [Google Scholar]
- Paller A, Pariser D, Siegfried E, Kricorian G. Safety and efficacy of etanercept treatment in children and adolescents with plaque psoriasis: 96‐week results of open‐label extension study. Journal of the American Academy of Dermatology 2010;3(Suppl 1):AB11. [EMBASE: 70142564] [Google Scholar]
- Paller A, Siegfried E, Langley R, Gottlieb A. A 12 week phase 3 study of efficacy and safety of etanercept therapy in children and adolescents with moderate to severe plaque psoriasis. Journal of the American Academy of Dermatology 2007;56(2):AB195. [Google Scholar]
- Paller AS, Eichenfield LF, Langley RG, Leonardi CL, Siegfried EC, Creamer K, et al. Subgroup analyses of etanercept in pediatric patients with psoriasis. Journal of the American Academy of Dermatology 2010;63(2):e38‐41. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Paller AS, Pariser D, Foehl J, Pedersen R, Molta C. Interim results of a long‐term safety and tolerability study of etanercept treatment in children and adolescents age 8 to 17 years with plaque psoriasis (Abstract). 10th ESPD Congress Lausanne Switzerland. European Journal of Pediatric Dermatology 2010;20(1):52‐3. [EMBASE: 70182399] [Google Scholar]
- Paller AS, Siegfried EC, Eichenfield LF, Pariser D, Langley RG, Creamer K, et al. Long‐term etanercept in pediatric patients with plaque psoriasis. Journal of the American Academy of Dermatology 2010;63(5):762‐8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Paller AS, Siegfried EC, Langley RG, Gottlieb AB, Pariser D, Landells I, et al. Etanercept treatment for children and adolescents with plaque psoriasis. The New England Journal of Medicine 2008;358(3):241‐51. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Siegfried E, Levy M, Jahreis A, Paller A. Etanercept in children and adolescents with psoriasis. Journal of the American Academy of Dermatology 2006;54(3 Suppl):AB218. [Google Scholar]
- Siegfried EC, Eichenfield LF, Paller AS, Pariser D, Creamer K, Kricorian G. Intermittent etanercept therapy in pediatric patients with psoriasis. Journal of the American Academy of Dermatology 2010;63(5):769‐74. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Alsuwaidan 2011 {published data only}
- Alsuwaidan SN. Childhood psoriasis: Analytic retrospective study in Saudi patients. Journal of the Saudi Society of Dermatology & Dermatologic Surgery 2011;15(2):57‐61. [EMBASE: 2011649967] [Google Scholar]
Beikert 2012 {published data only}
- Beikert FC, Augustin M, Radtke MA. Etanercept in juvenile psoriasis [Etanercept in der Therapie der juvenilen Psoriasis]. Hautarzt 2012;63(5):406‐10. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
References to ongoing studies
NCT01090427 {published data only}
- NCT01090427. A study of the safety and efficacy of ustekinumab in adolescent patients with psoriasis (CADMUS). clinicaltrials.gov/show/NCT01090427 (accessed 31 October 2014).
NCT01100034 {published data only}
- NCT01100034. Long‐term, prospective, observational cohort study of safety and effectiveness of pediatric psoriasis patients treated with etanercept in a naturalistic setting: a post‐authorization safety study (PASS). clinicaltrials.gov/ct2/show/NCT01100034 (accessed 31 October 2014).
NCT01251614 {published data only}
- EUCTR2009‐013072‐52‐DE. A multicentre, randomised, double‐dummy, double‐blind study evaluating two doses of adalimumab versus methotrexate (MTX) in paediatric subjects with chronic plaque psoriasis (Ps). clinicaltrialsregister.eu/ctr‐search/search?query=EUCTR2009‐013072‐52‐DE (accessed 31 October 2014).
- NCT01251614. A multicenter, randomized, double‐dummy, double‐blind study evaluating two doses of adalimumab versus methotrexate (MTX) in pediatric subjects with chronic plaque psoriasis (Ps). clinicaltrials.gov/ct2/show/NCT01251614 (accessed 31 October 2014).
- Papp K, Thaci D, Marcoux D, Weibel L, Unnebrink K, Williams D. Efficacy and safety of adalimumab versus methotrexate treatment in pediatric patients with severe chronic plaque psoriasis: results from the 16‐week randomized, double‐blind period of a phase 3 study [abstract #2970552]. 23rd World Congress of Dermatology 2015 Abstracts and Proceedings; 2015 June 8‐13; Vancouver. 2015. [978‐0‐9685848‐3‐5]
- Papp K, Williams D, Thaci D, Landells I, Unnebrink K. Study design and baseline characteristics from a phase 3, randomized, double‐blind study of adalimumab versus methotrexate treatment in pediatric patients with chronic plaque psoriasis (Abstract). 72nd Annual Meeting of the American Academy of Dermatology Denver, CO United States. Journal of the American Academy of Dermatology 2014;70(5 Suppl 1):AB190. [EMBASE: 71390847] [Google Scholar]
NCT01432249 {published data only}
- NCT01432249. Post marketing surveillance to observe safety and efficacy of enbrel in pediatric patients with psoriasis. clinicaltrials.gov/ct2/show/NCT01432249 (accessed 31 October 2014).
Additional references
Augustin 2010
- Augustin M, Glaeske G, Radtke MA, Christophers E, Reich K, Schäfer I. Epidemiology and comorbidity of psoriasis in children. British Journal of Dermatology 2010;162(3):633‐6. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Beattie 2006
- Beattie PE, Lewis‐Jones MS. A comparative study of impairment of quality of life in children with skin disease and children with other chronic childhood diseases. British Journal of Dermatology 2006;155(1):145–51. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Bell 1991
- Bell LM, Sedlack R, Beard CM, Perry HO, Michet CJ, Kurland LT. Incidence of psoriasis in Rochester, Minn, 1980‐1983. Archives of Dermatology 1991;127(8):1184‐7. [PUBMED: 1863076] [PubMed] [Google Scholar]
Benoit 2007
- Benoit S, Hamm H. Childhood psoriasis. Clinics in Dermatology 2007;25(6):555–62. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Blandizzi 2014
- Blandizzi C, Gionchetti P, Armuzzi A, Caporali R, Chimenti S, Cimaz R, et al. The role of tumour necrosis factor in the pathogenesis of immune‐mediated diseases. International Journal of Immunopathology & Pharmacology 2014;27(1 Suppl):1‐10. [EMBASE: 24774503] [DOI] [PubMed] [Google Scholar]
Bronckers 2015
- Bronckers IM, Paller AS, Geel MJ, Kerkhof PC, Seyger MM. Psoriasis in children and adolescents: diagnosis, management and comorbidities. Paediatric Drugs 2015 Jun 14 [Epub ahead of print]. [DOI] [PMC free article] [PubMed]
Burden 1999
- Burden AD. Management of psoriasis in childhood. Clinical & Experimental Dermatology 1999;24(5):341‐5. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Cai 2012
- Cai Y, Fleming C, Yan J. New insights of T cells in the pathogenesis of psoriasis. Cellular & Molecular Immunology 2012;9(4):302–9. [EMBASE: 2012386227] [DOI] [PMC free article] [PubMed] [Google Scholar]
Chiricozzi 2011
- Chiricozzi A, Guttman‐Yassky E, Suárez‐Fariñas M, Nograles KE, Tian S, Cardinale I, et al. Integrative responses to IL‐17 and TNF‐alpha in human keratinocytes account for key inflammatory pathogenic circuits in psoriasis. Journal of Investigative Dermatology 2011;131(3):677‐87. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Chiricozzi 2014
- Chiricozzi A, Saraceno R, Chimenti MS, Guttman‐Yassky E, Krueger JG. Role of IL‐23 in the pathogenesis of psoriasis: a novel potential therapeutic target?. Expert Opinion in Therapeutic Targets 2014;18(5):513‐25. [EMBASE: 2014263796] [DOI] [PubMed] [Google Scholar]
de Jager 2010a
- Jager ME, Jong EM, Kerkhof PC, Seyger MM. Efficacy and safety of treatments for childhood psoriasis: a systematic literature review. Journal of the American Academy of Dermatology 2010;62(6):1013‐30. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
de Jager 2010b
- Jager ME, Kerkhof PC, Jong EM, Seyger MM. A cross‐sectional study using the Children's Dermatology Life Quality Index (CDLQI) in childhood psoriasis: negative effect on quality of life and moderate correlation of CDLQI with severity scores. British Journal of Dermatology 2010;163(5):1099‐101. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Egger 1997
- Egger M, Smith GD, Schneider M, Minder C. Bias in meta‐analysis detected by a simple, graphical test. BMJ 1997;315(7109):629‐34. [EMBASE: 1997278531] [DOI] [PMC free article] [PubMed] [Google Scholar]
Elliott 2009
- Elliott M, Benson J, Blank M, Brodmerkel C, Baker D, Sharples KR, et al. Ustekinumab: lessons learned from targeting interleukin 12/23p40 in immune‐mediated diseases. Annals of the New York Academy of Sciences 2009;1182:97‐110. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
EMA 2011
- European Medicines Agency. Assessment report for Enbrel International Nonproprietary Name: Etanercept Procedure No. Type II variation EMEA/H/C/262/II/134. www.ema.europa.eu/docs/en_GB/document_library/EPAR_‐_Assessment_Report_‐_Variation/human/000262/WC500113064.pdf (accessed 5 March 2014).
EMA 2013
- European Medicines Agency. Committee for Medicinal Products for Human Use (CHMP). Enbrel (etanercept) Procedure No. EMEA/H/C/000262/A46/134CHMP assessment report for paediatric use studies submitted according to Article 46 of the Regulation (EC) No 1901/2006. www.ema.europa.eu/docs/en_GB/document_library/EPAR_‐_Assessment_Report_‐_Variation/human/000262/WC500137357.pdf (accessed 5 March 2014).
EMA 2015
- European Medicines Agency. Committee for Medicinal Products for Human Use (CHMP). Extension of indication variation assessment report. Humira (adalimumab). Procedure No. EMEA/H/C/000481/II/0134. www.ema.europa.eu/docs/en_GB/document_library/EPAR_‐_Assessment_Report_‐_Variation/human/000481/WC500186769.pdf (accessed 9 July 2015).
Fallon‐Friedlander 2002
- Fallon‐Friedlander S. Psoriasis and the pediatric practitioner: emerging therapies. Medscape Dermatology 2002;3:2. [Google Scholar]
Farber 1999
- Farber EM, Nall L. Childhood psoriasis. Cutis 1999;64(5):309‐14. [MEDLINE: ] [PubMed] [Google Scholar]
Ferrándiz 2002
- Ferrándiz C, Pujol RM, García‐Patos V, Bordas X, Smandía JA. Psoriasis of early and late onset: a clinical and epidemiologic study from Spain. Journal of the American Academy of Dermatology 2002;46(6):867‐73. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Gordon 2012
- Gordon KB, Langley RG, Gottlieb AB, Papp KA, Krueger GG, Strober BE, et al. A phase III, randomized, controlled trial of the fully human IL‐12/23 mAb briakinumab in moderate‐to‐severe psoriasis. Journal of Investigative Dermatology 2012;132(2):304‐14. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Gånemo 2011
- Gånemo A, Wahlgren CF, Svensson A. Quality of life and clinical features in Swedish children with psoriasis. Pediatric Dermatology 2011;28(4):375‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Harper 2009
- Harper EG, Guo C, Rizzo H, Lillis JV, Kurtz SE, Skorcheva I, et al. Th17 cytokines stimulate CCL20 expression in keratinocytes in vitro and in vivo: implications for psoriasis pathogenesis. Journal of Investigative Dermatology 2009;129(9):2175‐83. [EMBASE: 2009528443] [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Huerta 2007
- Huerta C, Rivero E, Rodríguez LA. Incidence and risk factors for psoriasis in the general population. Archives of Dermatology 2007;143(12):1559‐65. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Iborra 2011
- Iborra M, Beltrán B, Bastida G, Aguas M, Nos P. Infliximab and adalimumab‐induced psoriasis in Crohn's disease: a paradoxical side effect. Journal of Crohn's & Colitis 2011;5(2):157‐61. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Krueger 2002
- Krueger JG. The immunologic basis for the treatment of psoriasis with new biologic agents. Journal of the American Academy of Dermatology 2002;46(1):1‐23. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Krueger 2004
- Krueger G, Callis K. Potential of tumor necrosis factor inhibitors in psoriasis and psoriatic arthritis. Archives of Dermatology 2004;140(2):218‐25. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Landells 2010
- Landells I, Paller AS, Pariser D, Kricorian G, Foehl J, Molta C, et al. Efficacy and safety of etanercept in children and adolescents aged > or = 8 years with severe plaque psoriasis. European Journal of Dermatology 2010;20(3):323‐8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Langley 2011
- Langley RG, Paller AS, Hebert AA, Creamer K, Weng HH, Jahreis A, et al. Patient‐reported outcomes in pediatric patients with psoriasis undergoing etanercept treatment: 12‐week results from a phase III randomized controlled trial. Journal of the American Academy of Dermatology 2011;64(1):64‐70. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Lapadula 2014
- Lapadula G, Marchesoni A, Armuzzi A, Blandizzi C, Caporali R, Chimenti S, et al. Adalimumab in the treatment of immune‐mediated diseases. International Journal of Immunopathology & Pharmacology 2014;27(1 Suppl):33‐48. [EMBASE: 24774505] [DOI] [PubMed] [Google Scholar]
Leman 2001
- Leman J, Burden D. Psoriasis in children, a guide to its diagnosis and management. Paediatric Drugs 2001;3(9):673‐80. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Leonardi 2012
- Leonardi C, Matheson R, Zachariae C, Cameron G, Li L, Edson‐Heredia E, et al. Anti‐interleukin‐17 monoclonal antibody ixekizumab in chronic plaque psoriasis. The New England Journal of Medicine 2012;366(13):1190‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Levy 2005
- Levy ML, Jahreis A, Paller AS, Walker S, Molta C, Freundlich B. Etanercept in children and adolescents with psoriasis (Abstract P08. 119). The 14th Congress of the European Academy of Dermatology and Venereology, London, UK. 12‐15th October 2005. Journal of the European Academy of Dermatology & Venereology 2005;19(Suppl 2):251. [Google Scholar]
Lewis‐Jones 1995
- Lewis‐Jones MS, Finlay AY. The children’s dermatology life quality index (CDLQI): initial validation and practical use. British Journal of Dermatology 1995;132(6):942‐9. [PUBMED: 7662573] [DOI] [PubMed] [Google Scholar]
Liu 2006
- Liu GF, Wang J, Liu K, Snavely DB. Confidence intervals for an exposure adjusted incidence rate difference with applications to clinical trials. Statistics in Medicine 2006;25(8):1275‐86. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Marji 2010
- Marji JS, Marcus R, Moennich J, Mackay‐Wiggan J. Use of biologic agents in pediatric psoriasis. Journal of Drugs in Dermatology 2010;9(8):975‐86. [MEDLINE: ] [PubMed] [Google Scholar]
McCroskery 2010
- McCroskery P, Wallace CA, Lovell DJ, Stryker S, Chernyukhin N, Blosch C, et al. Summary of worldwide pediatric malignancies reported after exposure to etanercept. Pediatric Rheumatology Online Journal 2010;8:18. [PUBMED: 20546618] [DOI] [PMC free article] [PubMed] [Google Scholar]
Menter 2004
- Menter MA, Cush JM. Successful treatment of pediatric psoriasis with infliximab. Pediatric Dermatology 2004;21(1):87‐8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Mittal 2010
- Mittal M, Raychaudhuri SP. Golimumab and certolizumab: the two new anti‐tumor necrosis factor kids on the block. Indian Journal of Dermatology, Venereology & Leprology 2010;76(6):602‐8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
NICE 2012
- National Institute for Health and Clinical Excellence. Psoriasis assessment and management of psoriasis. Clinical guideline methods, evidence and recommendations. October 2012. www.nice.org.uk/guidance/cg153 (accessed 01 August 2015).
Paller 2007
- Paller A, Siegfried E, Langley R, Gottlieb A. A 12 week phase 3 study of efficacy and safety of etanercept therapy in children and adolescents with moderate to severe plaque psoriasis (Abstract P2783). American Academy of Dermatology 65th Annual Meeting February 2‐6, 2007. Journal of the American Academy of Dermatology 2007;56(2):AB195. [Google Scholar]
Paller 2010a
- Paller AS, Siegfried EC, Eichenfield LF, Pariser D, Langley RG, Creamer K, et al. Long‐term etanercept in pediatric patients with plaque psoriasis. Journal of the American Academy of Dermatology 2010;63(5):762‐8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Paller 2010b
- Paller AS, Eichenfield LF, Langley RG, Leonardi CL, Siegfried EC, Creamer K, et al. Subgroup analyses of etanercept in pediatric patients with psoriasis. Journal of the American Academy of Dermatology 2010;63(2):e38‐41. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Paller 2010c
- Paller AS, Pariser D, Foehl J, Pedersen R, Molta C. Interim results of a long‐term safety and tolerability study of etanercept treatment in children and adolescents age 8 to 17 years with plaque psoriasis (Abstract). 10th ESPD Congress Lausanne Switzerland. European Journal of Pediatric Dermatology 2010;20(1):52‐3. [EMBASE: 70182399] [Google Scholar]
Paller 2010d
- Paller A, Pariser D, Siegfried E, Kricorian G. Safety and efficacy of etanercept treatment in children and adolescents with plaque psoriasis: 96‐week results of open‐label extension study. Conference: 68th Annual Meeting of the American Academy of Dermatology, AAD Miami, FL United States. Conference Start: 20100305 Conference End: 20100309. Journal of the American Academy of Dermatology 2010;62(3 Suppl 1):AB11. [EMBASE: 70142564] [Google Scholar]
Papp 2012
- Papp KA, Leonardi C, Menter A, Ortonne JP, Krueger JG, Kricorian G, et al. Brodalumab, an anti‐interleukin‐17‐receptor antibody for psoriasis. The New England Journal of Medicine 2012;366(13):1181‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Papp 2014
- Papp K, Williams D, Thaci D, Landells I, Unnebrink K. Study design and baseline characteristics from a phase 3, randomized, double‐blind study of adalimumab versus methotrexate treatment in pediatric patients with chronic plaque psoriasis (Abstract). 72nd Annual Meeting of the American Academy of Dermatology Denver, CO United States. Journal of the American Academy of Dermatology 2014;70(5 Suppl 1):AB190. [EMBASE: 71390847] [Google Scholar]
Papp 2015
- Papp K, Thaci D, Marcoux D, Weibel L, Unnebrink K, Williams D. Efficacy and safety of adalimumab versus methotrexate treatment in pediatric patients with severe chronic plaque psoriasis: results from the 16‐week randomized, double‐blind period of a phase 3 study [abstract #2970552]. 23rd World Congress of Dermatology 2015 Abstracts and Proceedings; 2015 June 8‐13; Vancouver. 2015. [978‐0‐9685848‐3‐5]
Puig 2013
- Puig L. Induction phase, primary endpoint, time to decide on primary failure, and therapeutic goals in biologic treatment of psoriasis. Journal of the European Academy of Dermatology & Venereology 2013;27(2):e257‐60. [EMBASE: 2013055875] [DOI] [PubMed] [Google Scholar]
Ramsay 1991
- Ramsay B, Lawrence CM. Measurement of involved surface area in patients with psoriasis. British Journal of Dermatology 1991;124(6):565–70. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Raychaudhuri 2000
- Raychaudhuri SP, Gross J. A comparative study of pediatric onset psoriasis with adult onset psoriasis. Pediatric Dermatology 2000;17(3):174–8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
RevMan 2014 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Schafer 2012
- Schafer P. Apremilast mechanism of action and application to psoriasis and psoriatic arthritis. Biochemical Pharmacology 2012;83(12):1583‐90. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Seyhan 2006
- Seyhan M, Coskun BK, Saglam H, Ozcan H, Karincaoglu Y. Psoriasis in childhood and adolescence: evaluation of demographic and clinical features. Pediatrics International 2006;48(6):525‐30. [EMBASE: 2006580444] [DOI] [PubMed] [Google Scholar]
Siddiqui 2009
- Siddiqui O. Statistical methods to analyze adverse events data of randomized clinical trials. Journal of Biopharmaceutical Statistics 2009;19(5):889‐99. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Siegfried 2006
- Siegfried E, Levy M, Jahreis A, Paller A. Etanercept in children and adolescents with psoriasis (Abstract P2880). American Academy of Dermatology 64th Annual Meeting March 3‐7, 2006. Journal of the American Academy of Dermatology 2006;54(3 Suppl):AB218. [Google Scholar]
Siegfried 2010
- Siegfried EC, Eichenfield LF, Paller AS, Pariser D, Creamer K, Kricorian G. Intermittent etanercept therapy in pediatric patients with psoriasis. Journal of the American Academy of Dermatology 2010;63(5):769‐74. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Silverberg 2009
- Silverberg NB. Pediatric psoriasis: an update. Therapeutics & Clinical Risk Management 2009;5:849‐56. [PUBMED: 19898649] [DOI] [PMC free article] [PubMed] [Google Scholar]
Spuls 2010
- Spuls PI, Lecluse LL, Poulsen ML, Bos JD, Stern RS, Nijsten T. How good are clinical severity and outcome measures for psoriasis?: quantitative evaluation in a systematic review. Journal of Investigative Dermatology 2010;130(4):933‐43. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Sticherling 2011
- Sticherling M, Augustin M, Boehncke WH, Christophers E, Domm S, Gollnick H, et al. Therapy of psoriasis in childhood and adolescence ‐ a German expert consensus. Journal der Deutschen Dermatologischen Gesellschaft 2011;9(10):815‐23. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Tan 2010
- Tan JK, Wolfe BJ, Bulatovic R, Jones EB, Lo AY. Critical appraisal of quality of clinical practice guidelines for treatment of psoriasis vulgaris, 2006‐2009. Journal of Investigative Dermatology 2010;130(10):2389‐95. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Tollefson 2010
- Tollefson MM, Crowson CS, McEvoy MT, Maradit Kremers H. Incidence of psoriasis in children: a population‐based study. Journal of the American Academy Dermatology 2010;62(6):979‐87. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
US FDA 2009
- US Food, Drug Administration. Information for healthcare professionals: tumor necrosis factor (TNF) blockers (marketed as Remicade, Enbrel, Humira, Cimzia, and Simponi). www.fda.gov/Drugs/DrugSafety/PostmarketDrugSafetyInformationforPatientsandProviders/DrugSafetyInformationforHeathcareProfessionals/ucm174474.htm (accessed 5 March 2014).
US FDA 2012
- US Food, Drug Administration. Enbrel (etanercept) prescribing information December 2012. www.fda.gov/Safety/MedWatch/SafetyInformation/ucm334668.htm (accessed 5 March 2014).
Victor 2003
- Victor FC, Gottlieb AB, Menter A. Changing paradigms in dermatology: tumor necrosis factor alpha (TNF‐alpha) blockade in psoriasis and psoriatic arthritis. Clinics in Dermatology 2003;21(5):392‐7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Vogel 2012
- Vogel SA, Yentzer B, Davis SA, Feldman SR, Cordoro KM. Trends in pediatric psoriasis outpatient health care delivery in the United States. Archives of Dermatology 2012;148(1):66‐71. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Walsh 2002
- Walsh G. Biopharmaceuticals and biotechnology medicines: an issue of nomenclature. European Journal of Pharmaceutical Sciences 2002;15(2):135‐8. [PUBMED: 11849909] [DOI] [PubMed] [Google Scholar]
Weger 2010
- Weger W. Current status and new developments in the treatment of psoriasis and psoriatic arthritis with biological agents. British Journal of Pharmacology 2010;160(4):810‐20. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
References to other published versions of this review
Sanclemente 2012
- Sanclemente G, Murphy R, Contreras J, Rengifo‐Pardo M, Garcia H, Bonfill Cosp X. Anti‐TNF agents for paediatric psoriasis. Cochrane Database of Systematic Reviews 2012, Issue 8. [DOI: 10.1002/14651858.CD010017] [DOI] [PMC free article] [PubMed] [Google Scholar]


