SUMMARY
The mainstay of pharmacological treatment of overactive bladder (OAB) is anticholinergic therapy using muscarinic receptor antagonists (tertiary or quaternary amines). Muscarinic receptors in the brain play an important role in cognitive function, and there is growing awareness that antimuscarinic OAB drugs may have adverse central nervous system (CNS) effects, ranging from headache to cognitive impairment and episodes of psychosis. This review discusses the physicochemical and pharmacokinetic properties of OAB antimuscarinics that affect their propensity to cause adverse CNS effects, as observed in phase III clinical trials and in specific investigations on cognitive function and sleep architecture. PubMed/MEDLINE was searched for “OAB” plus “muscarinic antagonists” or “anticholinergic drug.” Additional relevant literature was identified by examining the reference lists of papers identified through the search. Preclinical and clinical trials in adults were assessed, focusing on the OAB antimuscarinics approved in the United States. The blood–brain barrier (BBB) plays a key role in protecting the CNS, but it is penetrable. The lipophilic tertiary amines, particularly oxybutynin, are more likely to cross the BBB than the hydrophilic quaternary amine trospium chloride, for which there are very few reports of adverse CNS effects. In fact, in 2008 the US product labels for oral oxybutynin were modified to include the potential for anticholinergic CNS events and a warning to monitor patients for adverse CNS effects. Even modest cognitive impairment in the elderly may negatively affect independence; therefore, selection of an antimuscarinic OAB drug with reduced potential for CNS effects is advisable.
Keywords: Antimuscarinic drugs, Blood–brain barrier, CNS effects, Overactive bladder
Introduction
Overactive bladder (OAB) is a chronic condition characterized by the lower urinary tract symptoms of urinary urgency, with or without urge incontinence, usually with urinary frequency and nocturia [1]. OAB affects approximately 17% of US adults, and there is a marked increase in prevalence with increasing age [2]. Although the prevalence among men and women in the United States is similar (16.0% vs. 16.9%, respectively), the severity and nature of symptom expression does differ, with women demonstrating a higher incidence of urge incontinence [2]. OAB has a significant impact on the health‐related quality of life, mental health, and quality of sleep of affected individuals whether or not they display the symptom of urge incontinence [2, 3]. The economic burden of OAB is also significant, estimated at approximately $12 billion per annum in the United States alone [4].
Symptoms of OAB are usually attributed to detrusor muscle overactivity during the bladder filling/urine storage phase, and may be neurogenic, myogenic, or idiopathic in nature [5, 6]. Detrusor smooth muscle activity is predominantly under the control of the parasympathetic system and involves acetylcholine acting on muscarinic receptors. Consequently, the mainstay of pharmacological treatment for OAB is anticholinergic therapy using muscarinic receptor antagonists, which have proven efficacy compared with placebo in relieving the symptoms of OAB [7, 8]. However, Herbison et al. [9] have suggested that the benefits are of limited clinical significance, with the extent of improvement versus placebo being similar to that achieved by nonpharmacological bladder retraining. A Cochrane database review that included 13 clinical trials comparing anticholinergic drugs with behavioral therapy for OAB found that there was more symptomatic improvement seen with anticholinergics compared with bladder training alone, and that combination treatment (anticholinergic drugs plus bladder training) was more effective than either modality alone [10]. Another recent study concluded that the addition of behavioral training to drug therapy may reduce incontinence frequency during active treatment but does not improve the ability to discontinue drug therapy and maintain improvement in urinary incontinence [11]. The following anticholinergic agents have been approved by the US Food and Drug Administration for the treatment of OAB; the tertiary amines oxybutynin, tolterodine, darifenacin, solifenacin, and fesoterodine, and the quaternary amine trospium chloride [6, 12].
All five muscarinic receptor subtypes have been identified in brain tissue, and adverse effects of anticholinergics on the central nervous system (CNS) may have important consequences for patients, particularly the elderly [13, 14], who are more likely to have impairment in the blood–brain barrier (BBB). Structural differences between the anticholinergics affect their pharmacokinetics, pharmacodynamics, and propensity to cause adverse effects, including those associated with the CNS [6, 12, 14, 15]. This article will review the pharmacological parameters that underlie the observed differences between antimuscarinic drugs in their propensity to adversely affect cognitive function, and data on CNS effects observed with the OAB antimuscarinics, based on both adverse event data from clinical trials and studies specifically designed to assess CNS effects. PubMed/MEDLINE was searched for records using the terms “OAB” plus “muscarinic antagonists” or “anticholinergic drug.” Additional relevant literature was identified by examining the reference lists of papers identified through the literature search. Preclinical and clinical trials in adults were assessed, with focus on the antimuscarinic drugs approved for treatment of OAB in the United States (darifenacin, fesoterodine, oxybutynin, solifenacin, tolterodine, and trospium chloride).
Pharmacology of Differing CNS Effects
A number of factors influence the CNS effects of anticholinergic agents, including their ability to penetrate the CNS and, once past the BBB, their affinity for muscarinic receptors in the CNS. These factors are detailed in Table 1[6, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30, 31, 32, 33, 34].
Table 1.
Factors contributing to the pharmacokinetic profiles of antimuscarinic drugs for OAB [6, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30, 31, 32, 33, 34].
| Property | Oxybutynin | Tolterodine | Trospium | Darifenacin | Solifenacin | Fesoterodine ER |
|---|---|---|---|---|---|---|
| Molecular weight | 393.9 (chloride) | 475.6 | 427.97 | 507.5 | 480.55 | 527.66 |
| Octanol:water/buffer distribution/partition coefficient at pH 7.4, LogD/LogPa | >3.3 | 1.83 | −1.22 | 2.7 | 1.69 | 0.74b |
| Charge | Neutral | Positive | Positive (highly polar) | Positive | NA | Positive |
| Metabolites contributing to clinical effect | Desethyl‐oxybutynin | 5‐hydroxymethyl‐tolterodine | None | None | None | 5‐hydroxymethyl‐tolterodine |
| Metabolizing enzymes | CYP3A4 | CYP2D6, CYP3A4 | Non‐CYP450 mechanism | CYP2D6, CYP3A4 | CYP3A4 | CYP2D6, CYP3A4 |
| Half‐life (h) | IR: 2–3; ER: 12–13; patch: 7–8; gel: 64c | IR: 2c,d; ER: 8c,d | IR: 18; ER: 36 | ER: 12c,d | 45–68 | ER: 7–9c,d |
| Relative muscarinic receptor selectivitye | ||||||
| M3 versus M1 | 1.5 | 0.9 | 1.5 | 9.2 | 2.5 | 1.1 |
| M3 versus M2 | 10.0 | 1.1 | 1.3 | 58.2 | 12.5 | 1.0 |
ER, extended release; IR; immediate release; NA, not available; OAB, overactive bladder.
aOctanol: water logD values are presented for all agents except trospium chloride (octanol: buffer logP). Lower values indicate lower lipophilicity; a negative value indicates higher aqueous solubility and low lipophilicity.
bValue for active metabolite 5‐hydroxymethyl tolterodine.
cTerminal elimination half‐life.
dIn extensive metabolizers.
Adapted from Abrams and Andersson, 2007 with permission from Drs Abrams and Andersson, and Wiley‐Blackwell.
Crossing the BBB
The BBB, formed by the endothelial cells that line cerebral capillaries, plays an important role in maintaining the microenvironment for reliable neuronal signaling. In contrast to peripheral capillary beds, the brain endothelial cells have contiguous tight junctions between cells, lack fenestrations, and exhibit very low levels of endocytosis/transcytosis (reviewed by Abbott et al.) [35]. In addition, brain endothelial cells are surrounded by a basal membrane and extracellular matrix, and associated pericytes and astrocyte feet‐ends also form part of the BBB, contributing to its barrier function (Figure 1) [36].
Figure 1.
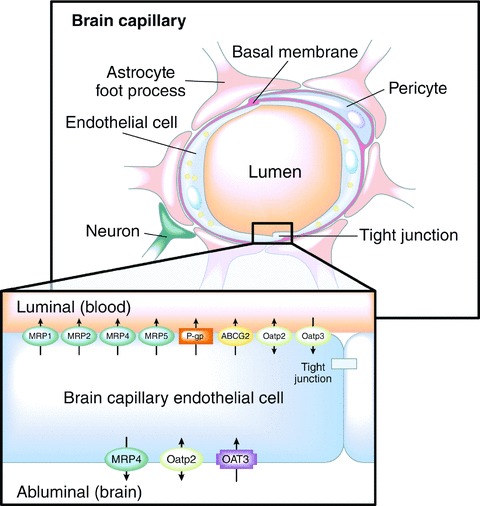
Schematic diagram of a brain capillary [36]. The BBB is formed by capillary endothelial cells, surrounded by a basal membrane and astrocyte foot processes. Tight junctions severely restrict the passage of water‐soluble, polar drugs across the BBB. Multiple drug efflux transporters (inset) further restrict the transcellular passage of certain lipid‐soluble drugs. MRP, multidrug resistance family of transporters; P‐gp, P‐glycoprotein; ABCG2, breast cancer resistance protein; Oatp, organic anion‐transporting polypeptide subfamily of anion transporters; OAT, organic anion and cation transporter. Arrows indicate the direction of translocation. Adapted from Deeken and Löscher, 2007 with permission from Drs Deeken and Löscher, and the American Association for Cancer Research, Inc.
The tight junctions effectively restrict paracellular passage of molecules between adjacent endothelial cells, including small ions such as Na+ and Cl−, resulting in high electrical resistance that may further restrict the entry of polar and ionic substances. Consequently, molecular transport across the BBB is forced to take a transcellular route. Small gaseous molecules such as O2 and CO2 can diffuse freely across the lipid membranes of the endothelium, as can small, lipophilic agents such as ethanol. Selective transport of required nutrients is mediated by specific transport systems on the luminal and abluminal endothelial membranes, and numerous efflux transporters have been identified within the BBB that serve to extrude any unwanted substrates back into the circulation that have managed to enter into the epithelial cell (Figure 1) [37]. The best characterized of these efflux pumps is P‐glycoprotein, a member of the multidrug resistance protein family [38]. Finally, various enzymes are found in the cytoplasm of endothelial cells, such as monoamine oxidase and cytochrome P450 (CYP1A and CYP2B), that can inactivate many neuroactive and toxic compounds [39]. These latter two mechanisms may, in part, explain why some small, lipophilic drugs demonstrate lower CNS permeability than would be expected from their physicochemical properties alone [35, 36].
Based on the physicochemical properties (molecular size, lipid solubility, and charge/polarity) of each of the antimuscarinic agents approved in the United States for use in the treatment of OAB (Table 1), one would predict that the tertiary amines have a higher propensity to cross the BBB than the quaternary amine trospium chloride. With its high lipophilicity, neutral charge, and low molecular weight, oxybutynin can readily penetrate the BBB. Furthermore, both oxybutynin and tolterodine are metabolized to produce molecules that contribute to the clinical effects of these drugs (Table 2) [16, 17, 18, 19, 20, 21, 22, 23], and CNS penetration of these active metabolites must also be considered. Although darifenacin is highly lipophilic, this molecule is a substrate for P‐glycoprotein efflux removal from the CNS [40].
Table 2.
CNS adverse events occurring in clinical studies as reported in product prescribing informationa[16, 17, 18, 19, 20, 21, 22, 23].
| Adverse CNS event | Percentage of patients | |||||||
|---|---|---|---|---|---|---|---|---|
| Oxybutynin | Tolterodine ER 4 mg/day | Trospium chloride ER 60 mg/day | Darifenacin 7.5 mg/day | Solifenacin 5−10 mg/day | Fesoterodine 4–8 mg/day | |||
| Oral ER 5−30 mg/day | Transdermal 3.9 mg/day | Gel 100 mg/day | ||||||
| Headache | 10.0 | NR | NR | 6.0 | NR | 6.7 | NR | NR |
| Dizziness | 6.0 | NR | 2.8 | 2.0 | NR | 0.9 | 1.8–1.9 | NR |
| Somnolence | 12.0 | NR | NR | 3.0 | <1.0 | NR | NR | NR |
| Anxiety/nervousness | <5.0b | NR | NR | 1.0 | NR | NR | NR | NR |
| Other | <5.0b,c | NR | NR | NR | NR | NR | 0.8–1.2d | 0.4–1.3e |
CNS, central nervous system; ER, extended release; NR, not reported.
aData summarized from respective Prescribing Information.
bAdverse events occurring in ≥1% to <5% of patients.
cConfusional state, dysgeusia, depression, insomnia.
dDepression.
eInsomnia.
Pharmacokinetic properties may also influence the propensity for CNS penetration, including serum levels and half‐lives of active metabolites, which, if high, may promote passive diffusion across the BBB. All of the tertiary amine OAB drugs are metabolized by the hepatic cytochrome P450 enzymes CYP2D6 and/or CYP3A4 [6]. Approximately 7% to 10% of Caucasians and 1% to 3% of African Americans and Asians are deficient in CYP2D6, which can lead to elevated serum levels of drugs that are metabolized, at least in part, by CYP2D6 (darifenacin, fesoterodine, and tolterodine) [17, 19, 21, 25]. Similarly, coadministration of drugs that inhibit CYP3A4 activity, such as macrolide antibiotics and azole antifungal agents, or inhibit CYP2D6 activity, such as fluoxetine, may also lead to elevated serum levels of the tertiary amines [16, 17, 19, 20, 21, 25]. Furthermore, oxidative metabolism via the CYP450 system declines with increasing age [41], which may contribute to the observed higher serum levels and/or prolonged elimination half‐life of some of these drugs in the elderly [16, 17, 19, 20, 25]. Nevertheless, although there are no data thus far to definitively indicate that such interactions influence CNS penetration and associated adverse effects in patients with OAB, it is worth noting that transdermal delivery of oxybutynin via patch or gel, which provides lower, more stable serum levels, is associated with lower rates of CNS adverse events than oral oxybutynin [15, 22, 23].
A number of factors contribute to the very low propensity for the quaternary amine trospium chloride to cross the BBB [27, 33, 42]. The fourth substitution on the nitrogen atom of trospium chloride gives the nitrogen a positive charge across all physiological pHs and increased hydrophilicity (decreased lipophilicity) compared with the tertiary amines (Table 1). Studies in mice suggest that the drug efflux transporter P‐glycoprotein is also involved in restricting the entry of trospium chloride, but not oxybutynin, into the brain [43, 44]. Furthermore, trospium chloride is not metabolized by the CYP450 system in the liver, thus minimizing the potential for elevated serum concentrations arising from drug–drug interactions and age‐ and genetic‐related impairment of the CYP450 system [27, 45, 46]. Indeed, trospium chloride is not extensively metabolized at all. Rather, 60% to 80% of the absorbed dose is excreted via the kidneys as unchanged compound [27, 45, 46]. Although trospium chloride has the potential to interact with other drugs that are eliminated by active tubular excretion [18], this is unlikely due to the large capacity for tubular secretion, except in those patients with severely impaired renal function.
A recent summary assessment of preclinical CNS access of the antimuscarinics exists [47]. Lipophilicity (octanol: water distribution test), permeability coefficient across porcine brain endothelial cell monolayers, and P‐glycoprotein substrate studies support a low propensity for the quaternary and a high propensity for the tertiary amines to cross the BBB.
CNS Accumulation and Receptor Selectivity
The permeability of the BBB is not uniform or stable throughout life and a number of conditions can impair its functionality including increasing age, use of certain medications, stress, and various comorbid diseases such as Alzheimer's and Parkinson's diseases, and type 2 diabetes mellitus [14, 48, 49]. Increased CNS penetration of otherwise excluded molecules may result from a number of dysfunctions including physical damage to the vascular basement membrane or extracellular matrix and disruption of tight junctions. Consequently, all the antimuscarinic OAB agents should be considered to have the potential to cross the compromised BBB and gain access to the CNS.
The bladder detrusor muscle contains predominantly M2 and M3 muscarinic receptors, although the M3 receptors are considered to be the most important for detrusor contraction; however, the afferent receptors are primarily M2≫M3. All five of the muscarinic receptors (M1−M5) are expressed in the brain, with varying distribution and abundance [14]. In particular, M1 receptors are abundant in the neocortex, hippocampus, and neostriatum, and, along with M2 receptors (present throughout the brain), are considered to play a major role in memory and cognitive function [14]. M3 receptors have relatively low expression in the brain. Drugs selective for the M3 receptor might be expected to have clinical efficacy in the treatment of OAB, while showing a reduced potential for adverse effects related to the blockade of other muscarinic receptor subtypes, such as CNS adverse effects [50].
CNS Effects of OAB Antimuscarinics
The adverse events most commonly associated with the use of antimuscarinic OAB drugs are the expected side effects of anticholinergics, such as dry mouth and constipation. CNS adverse events were generally observed in fewer than 10% of patients in phase III clinical trials, and were highest in patients receiving oral oxybutynin (Table 2) [16, 17, 18, 19, 20, 21], as would be expected from its propensity to cross the BBB. It should be noted that in 2008 the United States product labels for oral oxybutynin were modified to include the potential for anticholinergic CNS events and a warning to monitor patients for adverse CNS effects. Few CNS adverse events were observed for trospium chloride, the transdermal delivery forms of oxybutynin (patch and gel), and fesoterodine (Table 2).
The most commonly observed CNS adverse effects in patients receiving OAB drugs are headache, somnolence, and dizziness (Table 2); however, the spectrum of effects may also include cognitive impairment, confusion, fatigue, psychotic behavior, and insomnia [15, 51], and may range from mild symptoms such as drowsiness to severe intoxication manifested by hallucinations, severe cognitive impairment, and even coma. Hallucinations and episodes of psychosis following oral oxybutynin or tolterodine use have been reported [52, 53, 54]; several of the cases involving oral oxybutynin were reported in elderly patients with Parkinson's disease being treated for OAB [55], and case reports exist describing memory loss and impaired cognitive function associated with tolterodine use [56, 57]. Confusion and hallucinations have also been reported in association with solifenacin use in worldwide postmarketing experience, while in pivotal clinical trials, visual blurring (a marker of CNS penetration) was reported in up to 5% of subjects receiving solifenacin [20].
Level 1 evidence indicates that agents that prolong the presence of the neurotransmitter acetylcholine (e.g., donepezil, galantamine, and rivastigmine) demonstrate improvements in memory and cognition in patients with dementia [58, 59, 60, 61]. Agents that block the muscarinic receptor (e.g., oxybutynin and other OAB antimuscarinics) may, in effect, result in a pharmacodynamically induced Alzheimer's disease state.
Although adverse CNS effects can occur in patients of any age, the elderly are particularly vulnerable for a number of reasons, including an increased anticholinergic load and potential drug−drug interactions resulting from polypharmacy; age‐ and comorbid disease‐related decline in cholinergic function; impaired metabolic clearance of drugs; and impaired function of the BBB [15, 62, 63, 64, 65]. The cumulative anticholinergic clinical burden is not simple to measure and the clinical impact has not been well understood/characterized. In phase III randomized trials, there did not appear to be any increase in reports of CNS adverse events in elderly populations; this has been further demonstrated in studies of patients aged ≥65 years with tolterodine ER [66] and darifenacin [67], and in patients aged ≥75 years with trospium chloride ER [68].
However, caution is required when interpreting data from clinical studies. The incidence of CNS effects may be underreported because data are usually based on unsolicited reporting during protocol visits rather than formal neurological testing, and clinical studies often exclude those at higher risk such as elderly patients and those with cognitive impairment, or an existing anticholinergic drug load. Furthermore, it is recognized that patients may fail to report CNS symptoms because they may be unaware of cognitive impairment or changes in memory, or may assume it is related to aging, and not a drug effect [69]. Thus, it is important to consider data derived from clinical trials together with the results of studies specifically designed to investigate the CNS influence of antimuscarinic OAB drugs. A number of such studies have been conducted, using objective measures such as electroencephalographic (EEG) determination of CNS electrical activity and polysomnography. It should be noted that while neurocognitive measurements of memory have the benefit of being noninvasive, a potential limitation is that they may lack sensitivity. A new standard has been introduced in the form of studies measuring drug concentrations in the CNS and simultaneously performing clinical measurement of neurocognition and memory, such as the recently completed trospium chloride SMART (Sanctura Muscarinic Receptor Antagonist Resists Transport) trial.
Electroencephalographic Changes
EEG changes can serve as a marker for the effects of drugs on the CNS. Using quantitative EEG (qEEG), Pietzko et al. [70] demonstrated that oxybutynin induced significant power density decreases in the alpha1 and alpha2 frequency ranges and, to a lesser extent, in beta1 frequency in healthy volunteers. In contrast, there were no significant changes in any frequency range following oral trospium chloride. This work was confirmed and extended by Todorova et al. [71] who demonstrated that tolterodine, like trospium chloride, had negligible qEEG effects in healthy volunteers. The profound effects of oral oxybutynin were maintained throughout the observation period (at least 4 hours) [70, 71], and a clear cumulative effect was observed following multiple dose administration [71].
Cognitive Function, Memory, and Learning
Several studies have demonstrated that oral oxybutynin has a significant negative effect on cognitive function in healthy adults >65 years of age [69, 72, 73]. In one study, oxybutynin administration over 3 weeks significantly impaired delayed recall performance in the Name–Face Association Test, and the effect was comparable to the decline in memory function that occurs over 10 years in the normal aging process [69]. Darifenacin, in contrast, had no significant effect on cognitive function in healthy young [74] or elderly subjects [69, 75]. Similarly, a single dose of solifenacin did not significantly impair cognition compared with placebo in elderly subjects [73]. In the SMART trial, following administration of extended‐release trospium chloride 60 mg to elderly patients (aged 65–75 years) with OAB to achieve steady‐state plasma concentrations, trospium chloride assay was undetectable in the cerebrospinal fluid, and standardized memory testing showed no significant changes from baseline [76, 77].
Sleep Disturbances
Polysomnography was used to objectively determine the effect of single doses of antimuscarinic drugs on sleep architecture and duration in healthy volunteers without sleep‐related problems [78, 79]. Sleep architecture was influenced by some anticholinergics, and effects differed between that seen in healthy young volunteers (aged <36 years) [79] and older subjects (aged ≥50 years) [78]. In the study in younger volunteers, rapid eye movement (REM) sleep (duration as a percentage of total sleep time) was significantly reduced after oral oxybutynin compared with trospium chloride (18.4% vs. 20.2%; P < 0.05), although the reduction after oxybutynin compared with placebo and tolterodine (20.1% and 19.1%, respectively) did not reach significance [79]. In contrast, in the older volunteers, both oral oxybutynin and tolterodine significantly reduced REM sleep compared with placebo, while trospium chloride did not [78]. None of the study medications resulted in sleep disturbances of significant magnitude to impair cognitive function in younger or elderly volunteers [78, 79]. Data from these comparative studies show that oral oxybutynin reduced REM sleep in both younger and older volunteers, whereas tolterodine only reduced REM sleep in older volunteers, and trospium chloride did not affect REM sleep in either group. They suggest that, even with the potentially compromised BBB in older subjects, appreciable CNS changes are not seen with trospium chloride.
Somnolence/Sleepiness
A study in patients with OAB (mean age, 61 years) indicated that trospium chloride 20 mg twice daily did not increase daytime sleepiness or affect alertness levels, as determined using the self‐reported Stanford Sleepiness Scale, after 12 weeks of use [80]. Further analysis by age group (<65 or >65 years and <75 or >75 years) produced similar results.
Traffic Safety and Driving Alertness
Two studies compared the anticholinergic agents used to treat OAB in Germany in terms of safety issues related to driving. In the first study, Herberg and Fusgen compared the effects of therapeutic doses of trospium chloride, oral oxybutynin, and propiverine, a tertiary amine, on the ability to drive in traffic in 54 healthy volunteers in a randomized double‐blind trial [81]. After 7 days of treatment, no impairment in fitness to drive in traffic was noted with any of the agents. However, subjects treated with trospium chloride scored significantly higher than those receiving oxybutynin or propiverine on an assessment of vigilance, the readiness to react to changes seen on a computer monitor [81]. Overall, fewer adverse events were reported among those receiving trospium chloride compared with the other agents [81]. The results of this study were combined with those from a second randomized, double‐blind study that evaluated the general safety of tolterodine and trospium chloride compared with placebo in healthy volunteers treated for 8.5 days [82]. The combined analysis included a total of 108 subjects aged 30 to 70 years. Deterioration in overall traffic safety performance, measured by a battery of computer‐based performance tests, was not seen in any of the treatment groups at the beginning of treatment or at the therapeutic steady state [82]. However, paired comparisons evaluating the individual performance aspects at the prescribed measuring times indicated that tolterodine, propiverine, and oxybutynin led to impairment in tests of accuracy of perception, concentration, and vigilance, while trospium chloride did not [82]. The investigator concluded that trospium chloride offered advantages for traffic and overall safety over the other agents tested [82].
Implications for the Management of OAB
There is growing evidence from clinical studies and case reports to suggest that antimuscarinic treatment of OAB has the potential to cause clinically relevant impairment of CNS function—especially in the elderly neurocognitively vulnerable population. However, it is also clear that the antimuscarinic drugs used to treat OAB differ in their propensity to cause these effects, and the data support the hypothesis that the extent of CNS adverse effects can be explained in part by the ability of these drugs to cross the BBB and/or their muscarinic receptor specificity. Thus, the lipophilic tertiary amines, particularly oxybutynin, are more likely to cross the BBB than the quaternary amine trospium chloride, for which there are very few reports of adverse CNS effects. Efflux transporters limit CNS accumulation of both the tertiary amine darifenacin [40] and the quaternary amine trospium chloride [43, 44], which may account for the lower level of CNS effects for these drugs compared with other tertiary amines.
Adverse CNS effects are of particular concern in the treatment of OAB due to the high prevalence of OAB in the elderly. In certain elderly patients, even modest cognitive adverse effects, such as memory lapses and mild confusion, may lead to increased functional dependence and the inability to live completely independently [13, 64]. Furthermore, medications that increase cognitive impairment and cumulative anticholinergic load can exacerbate the risk of falls in elderly patients [54, 83, 84], which contribute a substantial component to the total cost burden of OAB treatment [85, 86]. Consequently, selection of an antimuscarinic drug with reduced potential for adverse CNS effects seems advisable, particularly for, but not limited to, elderly patients with OAB.
Conflict of Interest
The authors have no conflict of interest.
Disclosures
Michael B. Chancellor has disclosed that he serves as a consultant/advisor, investigator, and meeting participant/lecturer for Allergan, Inc; a consultant/advisor and meeting participant/lecturer for Astellas Pharma US, Inc; a consultant/advisor and investigator for Cook Medical; a board member/trustee for Lipella Pharmaceuticals Inc.; a meeting participant/lecturer and is involved in scientific studies/trials for Novartis Pharmaceuticals Corporation; a consultant/advisor, investigator, and meeting participant/lecturer for Pfizer, Inc; and is a consultant/advisor for Bayer HealthCare AG, Dainippon Sumitomo Pharma, and Xention, LTD.
Timothy B. Boone has disclosed that he serves as a consultant to Pfizer, Inc. and is a member of the speaker's bureau for Astellas Pharma US, Inc.
Author Contributions
Michael B. Chancellor contributed to the conception and design of the manuscript, was involved in drafting the outline and the critical review of the manuscript for scientific accuracy and factual content, and supervised the manuscript preparation.
Timothy B. Boone contributed to the conception and design of the manuscript and participated in the critical review and editing of the manuscript for scientific accuracy and factual content.
Acknowledgments
Rod McNab, Janet Manfre, and Sushma Soni of inScience Communications, a Wolters Kluwer business, provided editorial support, which was funded by Allergan, Inc.
References
- 1. Abrams P, Cardozo L, Fall M, et al The standardisation of terminology of lower urinary tract function: Report from the Standardisation Sub‐committee of the International Continence Society. Neurourol Urodyn 2002;21:167–178. [DOI] [PubMed] [Google Scholar]
- 2. Stewart WF, Van Rooyen JB, Cundiff GW, et al Prevalence and burden of overactive bladder in the United States. World J Urol 2003;20:327–336. [DOI] [PubMed] [Google Scholar]
- 3. Coyne KS, Payne C, Bhattacharyya SK, et al The impact of urinary urgency and frequency on health‐related quality of life in overactive bladder: Results from a national community survey. Value Health 2004;7:455–463. [DOI] [PubMed] [Google Scholar]
- 4. Hu TW, Wagner TH, Bentkover JD, et al Estimated economic costs of overactive bladder in the United States. Urology 2003;61:1123–1128. [DOI] [PubMed] [Google Scholar]
- 5. Chu FM, Dmochowski R. Pathophysiology of overactive bladder. Am J Med 2006;119:3–8. [DOI] [PubMed] [Google Scholar]
- 6. Abrams P, Andersson KE. Muscarinic receptor antagonists for overactive bladder. BJU Int 2007;100:987–1006. [DOI] [PubMed] [Google Scholar]
- 7. Chapple C, Khullar V, Gabriel Z, Dooley JA. The effects of antimuscarinic treatments in overactive bladder: A systematic review and meta‐analysis. Eur Urol 2005;48:5–26. [DOI] [PubMed] [Google Scholar]
- 8. Nabi G, Cody JD, Ellis G, Herbison P, Hay‐Smith J. Anticholinergic drugs versus placebo for overactive bladder syndrome in adults. Cochrane Database Syst Rev 2006;CD003781, doi: 10.1002/14651858.CD003781.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Herbison P, Hay‐Smith J, Ellis G, Moore K. Effectiveness of anticholinergic drugs compared with placebo in the treatment of overactive bladder: Systematic review. BMJ 2003;326:841–844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Alhasso AA, McKinlay J, Patrick K, Stewart L. Anticholinergic drugs versus non‐drug active therapies for overactive bladder syndrome in adults. Cochrane Database Syst Rev 2006:CD003193, doi: 10.1002/14651858.CD003193.pub3. [DOI] [PubMed] [Google Scholar]
- 11. Burgio KL, Kraus SR, Menefee S, et al Behavioral therapy to enable women with urge incontinence to discontinue drug treatment: A randomized trial. Ann Intern Med 2008;149:161–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Sussman DO. Overactive bladder: Treatment options in primary care medicine. J Am Osteopath Assoc 2007;107:379–385. [PubMed] [Google Scholar]
- 13. Feinberg M. The problems of anticholinergic adverse effects in older patients. Drugs Aging 1993;3:335–348. [DOI] [PubMed] [Google Scholar]
- 14. Abrams P, Andersson KE, Buccafusco JJ, et al Muscarinic receptors: Their distribution and function in body systems, and the implications for treating overactive bladder. Br J Pharmacol 2006;148:565–578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Staskin DR, Zoltan E. Anticholinergics and central nervous system effects: Are we confused? Rev Urol 2007;9:191–196. [PMC free article] [PubMed] [Google Scholar]
- 16. Ditropan XL. (oxybutynin chloride extended release tablets) Prescribing information. Raritan , NJ : Ortho‐McNeil Pharmaceutical, Inc; 2009. [Google Scholar]
- 17. Detrol LA. (tolterodine tartrate extended release capsules) Prescribing information. New York , NY : Pfizer, Inc; 2008. [Google Scholar]
- 18. Sanctura XR. (trospium chloride extended release capsules) Prescribing information. Irvine , CA : Allergan, Inc; 2008. [Google Scholar]
- 19. Enablex (darifenacin extended‐release tablets): Prescribing information. East Hanover , NJ : Novartis; 2008. [Google Scholar]
- 20. VESIcare (solifenacin succinate tablets) Prescribing information. Deerfield, IL and Research Triangle Park , NC : Astellas Pharma US Inc. and GlaxoSmithKline; 2008. [Google Scholar]
- 21. Toviaz (fesoterodine fumarate): Prescribing information. New York , NY : Pfizer, Inc; 2008. [Google Scholar]
- 22. Oxytrol (oxybutynin transdermal system) Prescribing information. Corona , CA : Watson Pharmaceuticals, Inc; 2006. [Google Scholar]
- 23. Gelnique (oxybutynin chloride gel) Prescribing information. Corona , CA : Watson Pharmaceuticals, Inc; 2009. [Google Scholar]
- 24. Ditropan (oxybutynin chloride tablets and syrup) Prescribing information. Raritan , NJ : Ortho‐McNeil Pharmaceutical, Inc; 2008. [Google Scholar]
- 25. Detrol (tolterodine tartrate tablets) Prescribing information. New York , NY : Pfizer, Inc; 2008. [Google Scholar]
- 26. Sanctura (trospium chloride 20 mg tablets) Prescribing information. Irvine , CA : Allergan, Inc; 2008. [Google Scholar]
- 27. Doroshyenko O, Jetter A, Odenthal KP, Fuhr U. Clinical pharmacokinetics of trospium chloride. Clin Pharmacokinet 2005;44:701–720. [DOI] [PubMed] [Google Scholar]
- 28. Nilvebrant L, Pahlman I, d’Argy R. Tissue distribution of tolterodine and its metabolites: Low penetration into the central nervous system. Eur Urol 2000;84. [Google Scholar]
- 29. Nilvebrant L, Gillberg PG, Sparf B. Antimuscarinic potency and bladder selectivity of PNU‐200577, a major metabolite of tolterodine. Pharmacol Toxicol 1997;81:169–172. [DOI] [PubMed] [Google Scholar]
- 30. Maniscalco M, Singh‐Franco D, Wolowich WR, Torres‐Colon R. Solifenacin succinate for the treatment of symptoms of overactive bladder. Clin Ther 2006;28:1247–1272. [DOI] [PubMed] [Google Scholar]
- 31. Ney P, Pandita RK, Newgreen DT, Breidenbach A, Stohr T, Andersson KE. Pharmacological characterization of a novel investigational antimuscarinic drug, fesoterodine, in vitro and in vivo. BJU Int 2008;101:1036–1042. [DOI] [PubMed] [Google Scholar]
- 32. Malhotra B, Gandelman K, Sachse R, Wood N, Michel MC. The design and development of fesoterodine as a prodrug of 5‐hydroxymethyl tolterodine (5‐HMT), the active metabolite of tolterodine. Curr Med Chem 2009;16:4481–4489. [DOI] [PubMed] [Google Scholar]
- 33. Pak RW, Petrou SP, Staskin DR. Trospium chloride: A quaternary amine with unique pharmacologic properties. Curr Urol Rep 2003;4:436–440. [DOI] [PubMed] [Google Scholar]
- 34. Ellsworth P, Kirshenbaum E. Update on the pharmacologic management of overactive bladder: The present and the future. Urol Nurs 2010;30:29–39, 53. [DOI] [PubMed] [Google Scholar]
- 35. Abbott NJ, Ronnback L, Hansson E. Astrocyte‐endothelial interactions at the blood‐brain barrier. Nat Rev Neurosci 2006;7:41–53. [DOI] [PubMed] [Google Scholar]
- 36. Deeken JF, Loscher W. The blood‐brain barrier and cancer: Transporters, treatment, and Trojan horses. Clin Cancer Res 2007;13:1663–1674. [DOI] [PubMed] [Google Scholar]
- 37. Golden PL, Pollack GM. Blood‐brain barrier efflux transport. J Pharm Sci 2003;92:1739–1753. [DOI] [PubMed] [Google Scholar]
- 38. Bauer B, Hartz AM, Fricker G, Miller DS. Modulation of p‐glycoprotein transport function at the blood‐brain barrier. Exp Biol Med (Maywood) 2005;230:118–127. [DOI] [PubMed] [Google Scholar]
- 39. el‐Bacha RS, Minn A. Drug metabolizing enzymes in cerebrovascular endothelial cells afford a metabolic protection to the brain. Cell Mol Biol (Noisy-le-grand) 1999;45:15–23. [PubMed] [Google Scholar]
- 40. Kay GG, Ebinger U. Preserving cognitive function for patients with overactive bladder: Evidence for a differential effect with darifenacin. Int J Clin Pract 2008;62:1792–1800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Tanaka E. In vivo age‐related changes in hepatic drug‐oxidizing capacity in humans. J Clin Pharm Ther 1998;23:247–255. [DOI] [PubMed] [Google Scholar]
- 42. Sandage B, Lerch G, Larsen G, Profy A. Trospium chloride does not cross the blood‐brain barrier of male Long Evans rats. Neurology 2009;72(Suppl 3):A50. [Google Scholar]
- 43. Geyer J, Gavrilova O, Petzinger E. The role of p‐glycoprotein in limiting brain penetration of the peripherally acting anticholinergic overactive bladder drug trospium chloride. Drug Metab Dispos 2009;37:1371–1374. [DOI] [PubMed] [Google Scholar]
- 44. Geyer J, Gavrilova O, Schwantes U. Differences in the brain penetration of the anticholinergic drugs trospium chloride and oxybutynin. UroToday Int J 2010;3, doi: 10.3834/uij.1944-5784.2010.02.12. [DOI] [Google Scholar]
- 45. Rovner ES. Trospium chloride in the management of overactive bladder. Drugs 2004;64:2433–2446. [DOI] [PubMed] [Google Scholar]
- 46. Chancellor MB, de Miguel F. Treatment of overactive bladder: Selective use of anticholinergic agents with low drug‐drug interaction potential. Geriatrics 2007;62:15–24. [PubMed] [Google Scholar]
- 47. Kay G, Malhotra B, Michel MC. Assessment of central nervous system access of a new antimuscarinic drug, fesoterodine. J Urol 2009;181(Suppl):84. [Google Scholar]
- 48. Starr JM, Wardlaw J, Ferguson K, MacLullich A, Deary IJ, Marshall I. Increased blood‐brain barrier permeability in type II diabetes demonstrated by gadolinium magnetic resonance imaging. J Neurol Neurosurg Psychiatry 2003;74:70–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Farrall AJ, Wardlaw JM. Blood‐brain barrier: Ageing and microvascular disease–systematic review and meta‐analysis. Neurobiol Aging 2009;30:337–352. [DOI] [PubMed] [Google Scholar]
- 50. Andersson KE. Potential benefits of muscarinic M3 receptor selectivity. Eur Urol Suppl 2002;1:23–28. [Google Scholar]
- 51. Klausner AP, Steers WD. Antimuscarinics for the treatment of overactive bladder: A review of central nervous system effects. Curr Urol Rep 2007;8:441–447. [DOI] [PubMed] [Google Scholar]
- 52. Gulsun M, Pinar M, Sabanci U. Psychotic disorder induced by oxybutynin: Presentation of two cases. Clin Drug Investig 2006;26:603–606. [DOI] [PubMed] [Google Scholar]
- 53. Tsao JW, Heilman KM. Transient memory impairment and hallucinations associated with tolterodine use. N Engl J Med 2003;349:2274–2275. [DOI] [PubMed] [Google Scholar]
- 54. Edwards KR, O’Connor JT. Risk of delirium with concomitant use of tolterodine and acetylcholinesterase inhibitors. J Am Geriatr Soc 2002;50:1165–1166. [DOI] [PubMed] [Google Scholar]
- 55. Donnellan CA, Fook L, McDonald P, Playfer JR. Oxybutynin and cognitive dysfunction. BMJ 1997;315:1363–1364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Salvatore S, Serati M, Cardozo L, Uccella S, Bolis P. Cognitive dysfunction with tolterodine use. Am J Obstet Gynecol 2007;197:e8. [DOI] [PubMed] [Google Scholar]
- 57. Womack KB, Heilman KM. Tolterodine and memory: dry but forgetful. Arch Neurol 2003;60:771–773. [DOI] [PubMed] [Google Scholar]
- 58. Rabins PV, Blacker D, Rovner BW, et al American Psychiatric Association practice guideline for the treatment of patients with Alzheimer's disease and other dementias. 2nd edition. Am J Psychiatry 2007;164:5–56. [PubMed] [Google Scholar]
- 59. Birks J. Cholinesterase inhibitors for Alzheimer's disease. Cochrane Database Syst Rev 2006:CD005593, doi: 10.1002/14651858.CD005593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Raina P, Santaguida P, Ismaila A, et al Effectiveness of cholinesterase inhibitors and memantine for treating dementia: Evidence review for a clinical practice guideline. Ann Intern Med 2008;148:379–397. [DOI] [PubMed] [Google Scholar]
- 61. Hansen RA, Gartlehner G, Webb AP, Morgan LC, Moore CG, Jonas DE. Efficacy and safety of donepezil, galantamine, and rivastigmine for the treatment of Alzheimer's disease: A systematic review and meta‐analysis. Clin Interv Aging 2008;3:211–225. [PMC free article] [PubMed] [Google Scholar]
- 62. Ancelin ML, Artero S, Portet F, Dupuy AM, Touchon J, Ritchie K. Non‐degenerative mild cognitive impairment in elderly people and use of anticholinergic drugs: Longitudinal cohort study. BMJ 2006;332:455–459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Kay GG, Abou‐Donia MB, Messer WS, Jr. , Murphy DG, Tsao JW, Ouslander JG. Antimuscarinic drugs for overactive bladder and their potential effects on cognitive function in older patients. J Am Geriatr Soc 2005;53:2195–2201. [DOI] [PubMed] [Google Scholar]
- 64. Kay GG, Granville LJ. Antimuscarinic agents: Implications and concerns in the management of overactive bladder in the elderly. Clin Ther 2005;27:127–138; quiz 139–140. [DOI] [PubMed] [Google Scholar]
- 65. Scheife R, Takeda M. Central nervous system safety of anticholinergic drugs for the treatment of overactive bladder in the elderly. Clin Ther 2005;27:144–153. [DOI] [PubMed] [Google Scholar]
- 66. Zinner NR, Mattiasson A, Stanton SL. Efficacy, safety, and tolerability of extended‐release once‐daily tolterodine treatment for overactive bladder in older versus younger patients. J Am Geriatr Soc 2002;50:799–807. [DOI] [PubMed] [Google Scholar]
- 67. Foote J, Glavind K, Kralidis G, Wyndaele JJ. Treatment of overactive bladder in the older patient: Pooled analysis of three phase III studies of darifenacin, an M3 selective receptor antagonist. Eur Urol 2005;48:471–477. [DOI] [PubMed] [Google Scholar]
- 68. Sand PK, Johnson TM II, Rovner ES, Ellsworth PI, Oefelein MG, Staskin DR. Trospium chloride once‐daily extended release is efficacious and tolerated in elderly subjects (aged >/= 75 years) with overactive bladder syndrome. BJU Int 2011;107:612–620. [DOI] [PubMed] [Google Scholar]
- 69. Kay G, Crook T, Rekeda L, et al Differential effects of the antimuscarinic agents darifenacin and oxybutynin ER on memory in older subjects. Eur Urol 2006;50:317–326. [DOI] [PubMed] [Google Scholar]
- 70. Pietzko A, Dimpfel W, Schwantes U, Topfmeier P. Influences of trospium chloride and oxybutynin on quantitative EEG in healthy volunteers. Eur J Clin Pharmacol 1994;47:337–343. [DOI] [PubMed] [Google Scholar]
- 71. Todorova A, Vonderheid‐Guth B, Dimpfel W. Effects of tolterodine, trospium chloride, and oxybutynin on the central nervous system. J Clin Pharmacol 2001;41:636–644. [DOI] [PubMed] [Google Scholar]
- 72. Katz IR, Sands LP, Bilker W, DiFilippo S, Boyce A, D’Angelo K. Identification of medications that cause cognitive impairment in older people: The case of oxybutynin chloride. J Am Geriatr Soc 1998;46:8–13. [DOI] [PubMed] [Google Scholar]
- 73. Wesnes KA, Edgar C, Tretter RN, Bolodeoku J. Exploratory pilot study assessing the risk of cognitive impairment or sedation in the elderly following single doses of solifenacin 10 mg. Expert Opin Drug Saf 2009;8:615–626. [DOI] [PubMed] [Google Scholar]
- 74. Kay GG, Wesnes KA. Pharmacodynamic effects of darifenacin, a muscarinic M selective receptor antagonist for the treatment of overactive bladder, in healthy volunteers. BJU Int 2005;96:1055–1062. [DOI] [PubMed] [Google Scholar]
- 75. Lipton RB, Kolodner K, Wesnes K. Assessment of cognitive function of the elderly population: Effects of darifenacin. J Urol 2005;173:493–498. [DOI] [PubMed] [Google Scholar]
- 76. Staskin D, Kay G, Tannenbaum C, et al Trospium chloride has no effect on memory testing and is assay undetectable in the central nervous system of older patients with overactive bladder. Int J Clin Pract 2010;64:1294–1300. [DOI] [PubMed] [Google Scholar]
- 77. Staskin D, Kay G, Tannenbaum C, et al Trospium chloride is undetectable in the older human central nervous system (CNS). J Am Geriatr Soc 2010;58:1618–1619. [DOI] [PubMed] [Google Scholar]
- 78. Diefenbach K, Arold G, Wollny A, Schwantes U, Haselmann J, Roots I. Effects on sleep of anticholinergics used for overactive bladder treatment in healthy volunteers aged > or = 50 years. BJU Int 2005;95:346–349. [DOI] [PubMed] [Google Scholar]
- 79. Diefenbach K, Donath F, Maurer A, et al Randomised, double‐blind study of the effects of oxybutynin, tolterodine, trospium chloride and placebo on sleep in healthy young volunteers. Clin Drug Investig 2003;23:395–404. [DOI] [PubMed] [Google Scholar]
- 80. Staskin DR, Harnett MD. Effect of trospium chloride on somnolence and sleepiness in patients with overactive bladder. Curr Urol Rep 2004;5:423–426. [DOI] [PubMed] [Google Scholar]
- 81. Herberg K, Fusgen I. Influence of trospium chloride, oxybutynin HCL and propiverine HCl on performances having relevance for safety. Geriat Fersch 1997;7:77–82. [Google Scholar]
- 82. Herberg K. Safety in everyday situations and street traffic with the use of medication for incontinence: New investigations into the safety and potential of urological anticholinergic drugs. Die Medizinsche Welt 1999;50:217–222. [Google Scholar]
- 83. Brown JS, Vittinghoff E, Wyman JF, et al Urinary incontinence: Does it increase risk for falls and fractures? Study of Osteoporotic Fractures Research Group. J Am Geriatr Soc 2000;48:721–725. [DOI] [PubMed] [Google Scholar]
- 84. Nebes RD, Pollock BG, Halligan EM, Kirshner MA, Houck PR. Serum anticholinergic activity and motor performance in elderly persons. J Gerontol A Biol Sci Med Sci 2007;62:83–85. [DOI] [PubMed] [Google Scholar]
- 85. Darkow T, Fontes CL, Williamson TE. Costs associated with the management of overactive bladder and related comorbidities. Pharmacotherapy 2005;25:511–519. [DOI] [PubMed] [Google Scholar]
- 86. Wagner TH, Hu TW, Bentkover J, et al Health‐related consequences of overactive bladder. Am J Manag Care 2002;8:S598–S607. [PubMed] [Google Scholar]


