Summary
Aims
Endothelial progenitor cells (EPCs) are involved in vascular repair and homeostasis after vascular injuries. In this study, we aimed to explore whether bone marrow (BM)‐derived EPCs contribute to neointima formation and reendothelialization in rabbit elastase‐induced aneurysm after flow diverter treatment.
Methods
Elastase‐induced aneurysms were created in New Zealand male rabbits. Three weeks after model creation, flow diverter was implanted to cover the induced aneurysm neck. Autologous EPCs were isolated from bone marrow, expanded ex vivo, double labeled with Hoechst 33,342 and CFSE(carboxyfluorescein diacetate succinimidyl ester), and transplanted transvenously into the rabbits. The rabbits were assigned into three groups. The first group received autologous transfusion of double‐labeled EPCs from the first day after stent implantation, and the second group received transfusion from the fifteenth day. The autologous transfusion was given at a 3‐day interval and continued for 2 weeks. Fluorescence‐labeled cells were tracked under fluorescence microscope at the aneurysm neck and parent artery in the two groups. The third group was established as control group without EPCs transplantation. Scanning electron microscope was used to investigate the reendothelialization rate between the former two groups and the control group.
Results
In the first group, double‐positive EPCs were found in 3/5 rabbits and mainly located in the subendothelial space and around the stent struts. In the second group, double‐positive EPCs were found in 2/5 rabbits and mainly located on the surface of neointima. More endothelial‐like cells were observed on the neointima of aneurysm neck and stented parent artery in the groups with EPCs transplantation than control group without EPCs transplantation, but the difference on the number of these cells did not reach statistical significance.
Conclusions
BM‐derived EPCs participate in neointima formation and reendothelialization in elastase‐induced aneurysm after flow diverter treatment. The EPCs may differentiate into different cell types according to the stages of neointima formation in vivo.
Keywords: Aneurysm, Endothelial progenitor cells, Flow diverter, Neointima
Introduction
Approximately 85% of patients with subarachnoid hemorrhage had ruptured intracranial aneurysms 1. In the past decades, surgical clipping was widely used as a traditional therapy for intracranial aneurysms. With the advancement of interventional technology, endovascular treatment has been widely accepted for its lower mortality and morbidity after surgical clipping of ruptured aneurysms 2. However, endovascular treatment faces a higher risk of recurrent bleeding than surgical clipping 3. Recently, flow diverter (FD) has been applied to treat intracranial aneurysms 4. The device can reconstruct the parent vessel lumen, provide a scaffold for neointima formation over the aneurysm neck, and eliminate the aneurysm from the systemic circulation. During the healing process, neointima formation is critical to avoid recurrence. Unfortunately, little is known about the mechanism of neointima formation at the aneurysm neck.
In 1997, Asahara et al. 5 found that CD34+ hematopoietic progenitor cells differentiated to endothelial cells (ECs) in vitro and may contribute to neoangiogenesis in vivo. The cells expressing CD34 and Flk‐1 are named as endothelial progenitor cells (EPCs). Since then, increasing data showed that EPCs were involved in vascular repair and homostasis 6, 7. Previously, we found that bone marrow‐derived EPCs were involved in aneurysm repair in rabbits after in situ or intravenous injection 8. However, it still remains unknown whether bone marrow (BM)‐derived EPCs contribute to neointima formation and reendothelialization in rabbit elastase‐induced aneurysm after FD treatment. In this study, we aimed to evaluate the role of EPCs in rabbit elastase‐induced aneurysm after FD treatment.
Materials and Methods
Animals and Study Design
New Zealand male rabbits (weight: 2.2–2.6 kg) were purchased from the Laboratory Animal Center of Shanghai Second Military Medical University. Animals were given food and water ad libitum, and all animal experiments were undertaken in accordance with the principles and guidelines of the Chinese Convention on Use of Laboratory Animals.
Sixteen New Zealand male rabbits were divided into three groups (first group, 5; second group, 5; control group, 6). Saccular aneurysms were induced with porcine pancreatic elastase. At 4 weeks after aneurysm establishment, FD was implanted. To investigate whether BM‐derived EPCs contribute to aneurysm repair after FD treatment, fluorescence‐labeled BM‐EPCs were autologously transfused into the model after FD treatment and were tracked under fluorescence microscope. To investigate the distribution of transfused cells in the neointima at different stages, the first group received transfusion of the labeled EPCs on day 1 after stent implantation, and the second group received transfusion of the labeled EPCs on day 15 after stent implantation. Control group without autologous EPCs transfusion was established to investigate whether transfused EPCs accelerate the reendothelialization in rabbit saccular aneurysm 2 weeks and 4 weeks after FD treatment.
Rabbit Model of Elastase‐Induced Aneurysms
Saccular aneurysm was induced under sodium pentobarbital anesthesia by using our modified technique as previously described 9. The right common carotid artery 2 cm proximal to the origin was ligated after placement of a temporary arcuate aneurysm clip at the origin. The proximal ligated lumen was digested endovascularly with a total of 75‐U porcine pancreatic elastase injected via a 22‐gauge catheter. The aneurysm clip was removed after ligation of the puncture point for catheter introduction. After the model establishment, heparin saline was administered intravenously for 3 days at a dose of 200 U/kg per day. Three weeks later, flow diversion stent (Tubridge; Microport, China) was implanted to cover the induced aneurysm, which was braided with 40% metal coverage. Antiplatelet treatment (aspirin, 10 mg/kg/day; clopidogrel, 10 mg/kg/day) was given by gavage for 3 days before stent implantation and continued after stent implantation until tissue harvest.
Autologous Isolation, Expansion, and Transfusion of EPCs
Fresh bone marrow was autologously obtained by iliac crest aspiration under general anesthesia. BM‐derived EPCs were isolated with Ficoll gradient centrifugation, plated on 25 cm2 fibronectin‐coated flasks, cultured in endothelial growth medium−2 MV (PromoCell, Heidelberg, Germany), and maintained for 7 days until expansion. EPCs were identified as adherent cells positive for CD133, CD34, vascular endothelial growth factor receptor II staining and for double staining with FITC‐UEA‐I (fluorescein isothiocyanate‐ulex europaeus agglutinin I, Sigma, St. Louis, MO, USA) and DiI‐acLDL (1,1′‐dioctadecyl‐3,3,3′,3′‐tetramethylindocarbocyanine perchlorate‐labeled AcLDL, Molecular Probes, Eugene, OR, USA) under fluorescence microscope. EPCs at the second to fourth passage were harvested at day 14, 17, and 20 of culture, double labeled with the DNA‐specific fluorochrome Hoechst 33,342 (1 μg/mL, Sigma, USA) for one hour and the intracellular fluorescent dye CFSE (carboxyfluorescein diacetate succinimidyl ester, 10 μmoL/mL, Santa Cruz Biotechnology, Santa Cruz, CA, USA) for 15 min at 37°C, suspended in 10 mL saline, and autologously transfused via the margin ear vein. The transfusion was given every 3 days in the first and second group and was totally performed for three times within 2 weeks.
Tissue Harvest and Processing
Animals were sacrificed with an overdose of sodium pentobarbital 2 weeks after the first transfusion of EPCs. The tissues in the first group and of three in control group were harvested 2 weeks after FD treatment, and the second group and the remained three in control group were harvested 4 weeks after FD treatment. The left ventricle was sequentially perfused with heparin and 4% paraformaldehyde before tissue harvesting. The implanted parent artery was longitudinally cut into two parts; one was stored at −80°C for EPCs tracking after stent strut removal, and the other was fixed in 4% paraformaldehyde or 2.5% glutaraldehyde for histomorphological analysis.
Cell Tracking and Histomorphological Analysis
The frozen tissues were molded in OCT and cut into 3‐μm‐thick tissue longitudinal sections. Identification of positive labeled cells was performed by fluorescence microscopic examination, which showed blue emission fluorescence for the labeling with Hoechst 33,342 and yellow emission fluorescence for the labeling with CFSE. For immunohistochemistry, the paraformaldehyde‐fixed tissue was paraffin‐embedded after removal of stent struts and cut into 3‐μm‐thick tissue longitudinal sections. To investigate the components of the neointima, these sections were stained with von Willebrand factor (vWF), CD68, and α‐actin (Santa Cruz Biotechnology). To investigate the reendothelialization at the sites of aneurysm neck, the stent‐implanted parent artery was fixed in 2.5% glutaraldehyde and processed for scanning electron microscope (SEM) and transmission electron microscope (TEM) examination.
Statistical Analysis
Results are presented as mean ± SD. The number of transfused EPCs between the first and second group was compared with unpaired t‐test, the number of endothelial‐like cells observed under SEM on the neointima, and stented parent arteries between the former two groups and control group were compared with unpaired t‐test. A value of P < 0.05 was considered significant.
Results
Elastase‐Induced Aneurysms and Flow Diverter Implantation
Saccular aneurysm was successfully induced with elastase in sixteen New Zealand male rabbits. The mean diameter of the sixteen aneurysms was 2.84 ± 0.8 mm in neck, 3.89 ± 0.66 mm in width, and 5.25 ± 2.18 mm in height. The median size of the stent was 3.5 mm in diameter and 14 mm in length. Exact deployment over the aneurysm neck was successfully performed in all rabbits. The induced aneurysms were completely occluded 2 weeks after stent implantation (Figure 1A–B). Hematoxylin‐eosin (HE) stain showed that a layer of neointima was covered on the parent artery and aneurysm neck (Figure 1C‐D).
Figure 1.
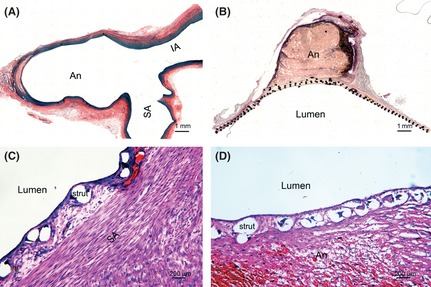
Neointima is formed on the aneurysm neck and parent artery after FD treatment. Histology samples stained with Victoria Blue demonstrate that saccular aneurysm is induced with elastase digestion in the ligated lumen of the right common carotid artery (A). H–E stain shows that aneurysm is occluded at 2 weeks after FD treatment (B), and the neointima is formed on the subclavian artery (C) and aneurysm neck (D). An, aneurysm; SA, subclavian artery; IA, innominate artery.
Quantities of BM‐Derived EPCs for Autologous Transplantation
Most cultures reached 80% confluence by 7 days and yielded 1.51 × 106 cells by 12 days. Both groups received autologous transfusion three times. The first group sequentially received 0.84 × 106, 0.83 × 106, 1.62 × 106 EPCs, and the second group sequentially received 0.80 × 106, 0.80 × 106, 1.63 × 106 EPCs. There was no statistical difference in the number of transfused EPCs between two groups (P > 0.05).
Distribution of Autologously Transplanted BM‐Derived EPCs
In the first group, double‐positive EPCs were detected in 3 out of 5 rabbits and mainly distributed in the subendothelium space (Figure 2A‐B) or around the stent struts (Figure 2C‐D). In the second group, double‐positive EPCs were detected in 2 of 5 rabbits and mainly distributed on the surface of intima (Figure 2E‐H).
Figure 2.
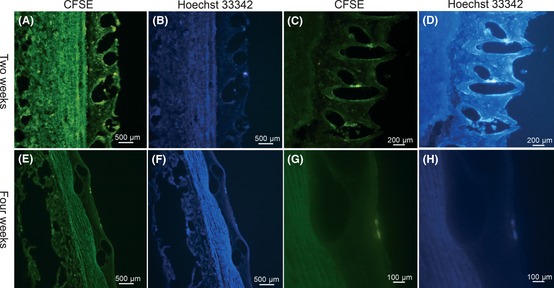
BM‐derived EPCs are tracked in neointima. In the early 2 weeks after autologous transfusion, EPCs with double fluorescence are observed in the subendothelium space (A–B) and around the stent struts(C–D). In the late 2 weeks after autologous transfusion, EPCs with double fluorescence are observed on the surface of neointima (E–H). Double‐positive cells were identified by showing yellow emission fluorescence for CFSE and blue emission fluorescence for Hoechst 33,342 concurrently.
Reendothelialization of the Aneurysm Neck after Flow Diverter Implantation
In the first group, scanning electron microscope showed that the aneurysm neck was fully covered by the neointima. Few EC‐like cells were observed in the neointima on the aneurysm neck and parent artery (Figure 3A‐B). Many scattered spider‐like platelets and inflammatory cells were found on the surface of neointima.
Figure 3.
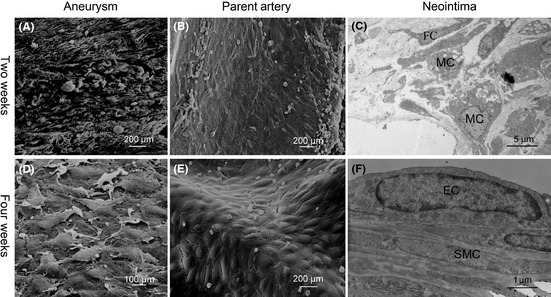
Neointima morphology is different at 2 weeks and 4 weeks after FD treatment. At 2 weeks after FD treatment, SEM shows that few ECs are attached on the surface of aneurysm neck (A) and the parent artery (B). TEM shows that most of the neointima is comprised of SMCs, MCs, and FCs with disorder (C). At 4 weeks after FD treatment, SEM shows that ECs emerge on the surface of the aneurysm neck (D) and parent artery (E). TEM shows that most of neointima is comprised of ECs and SMCs with order (F). EC, endothelial cell; SMC, smooth muscle cell; MC, macrophage cell; FC, fibroblast cell.
In the second group, SEM showed that lots of EC‐like cells were distributed in the neointima of aneurysm neck and parent artery (Figure 3D‐E), and few spider‐like platelets and inflammatory cells adhered to the neointima.
Compared with the control group, SEM results showed that all three groups had few EC‐like cells on the neointima of aneurysm 2 weeks after FD treatment, and the groups with autologous EPCs transfusion had more EC‐like cells on the surface of neointima at the aneurysm neck (33 vs. 29 cells/high‐power field, P > 0.05) and parent artery (94 vs. 85 cells/high‐power field, P > 0.05) than control group without EPCs transfusion 4 weeks after FD treatment.
Components of Neointima at the Aneurysm Neck
Two weeks after FD treatment, transmission electron microscope showed that cells in the neointima were mainly comprised of macrophages, smooth muscle cells (SMCs), and fibroblast cells (FC), and these cells were arranged in disarray (Figure 3C). Immunochemistry results demonstrated that cells with positive α‐actin staining were located in the subendothelium space (Figure 4A), macrophages with positive CD68 staining were located around the stent struts (Figure 4B), and the cells with positive vWF staining were seldom observed on the surface of the neointima (Figure 4C).
Figure 4.
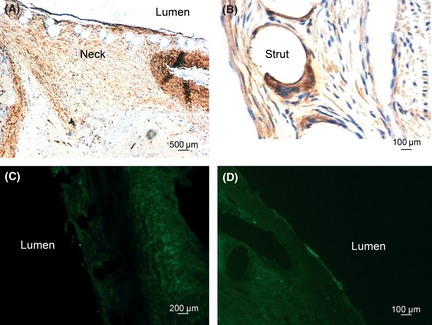
Histological analysis of neointima at 2 weeks and 4 weeks after FD treatment. At 2 weeks after FD treatment, immunochemistry shows that cells with positive α‐actin staining are distributed in the neointima of the aneurysm neck (A); cells with positive CD68 staining are located around the stent struts (B). Few cells with positive vWF staining are observed on the surface of neointima (C). At 4 weeks after FD treatment, cells with positive vWF staining are observed on the surface of neointima (D).
Four weeks after FD treatment, cells in the neointima were mainly comprised of ECs and SMCs with few macrophages, and these cells were arranged in order (Figure 3F). Immunochemistry results demonstrated that cells with positive vWF staining were distributed on the surface of the neointima (Figure 4D).
Discussion
We previously demonstrated that BM‐derived EPCs were involved in aneurysm wall repair after in situ or intravenous injection 8. In this successive study, we show that BM‐derived EPCs participate in the neointima formation in rabbit elastase‐induced aneurysms after FD treatment. We also observed that the double‐positive EPCs were distributed differently in the neointima when transfused at the early 2 weeks and the late 2 weeks. Our results indicated that BM‐derived EPCs probably differentiated into different cell types according to the stages of neointima formation.
Previously, repair of vascular injury was thought to rely on the migration of cells from the adjacent normal vascular wall 10. Recently, accumulating evidence shows that EPCs contribute to vascular repair and reendothelialization after arterial injuries 11, 12, 13, 14, 15, 16, 17. BM progenitor‐derived cells were found to be recruited in the wall of murine elastase saccular aneurysms 18. Transplantation of EPCs into denuded vessels was reported to accelerate reendothelialization and to attenuate neointima formation in injured vessels 19, 20. After mobilization of EPCs with granulocyte colony‐stimulating factor or statins, reendothelialization could be accelerated with reduced neointimal formation after vascular injury 14, 21, 22. The current study also shows that transplanted BM‐derived EPCs participate in the neointima formation in rabbits with elastase‐induced aneurysm after FD treatment.
Some studies maintained that BM‐derived EPCs did not contribute to the reendothelialization after vascular injuries 23, 24, 25. Tsuzuki 23 observed that BM‐derived EPCs were not involved in reendothelialized endothelium after simple endothelial denudation in mice. Our study also demonstrated that labeled EPCs were not tracked in all models. Many factors influence the tracking results of transplanted EPCs. Multiple types of progenitors, including adventitial EPCs and non‐BM‐derived EPCs, are involved in the process of vascular repair after arterial injuries 26, 27. It remains unclear, how these progenitors coordinate their function for the injured vessels. Furthermore, the contribution of BM‐derived cells to the cellular compartment of the neointima is limited to a transient period of the inflammatory response 28. Therefore, tracking time points should cover the stages of neointima formation and reendothelialization. In the current study, two time points at a two‐week interval were purposely selected to observe the role of EPCs during the process of neointima formation and reendothelialization. In addition, the labeled fluorescence gradually becomes weakened with time after cell differentiation 29, which further reduces the tracking sensitivity for transplanted EPCs. The results of our study showed that more endothelial‐like cells were observed on the neointima of aneurysm neck and parent artery with EPCs transplantation than without EPCs transplantation, and there were no significant difference on the number of endothelial‐like cells among the groups. Previously, He et al. 30 locally delivered fluorescence‐labeled EPCs in denuded carotid arteries of rabbits to investigate the effect of EPCs on the endothelial regeneration and found that the delivered EPCs can accelerate endothelialization. Different from their transplantation method, the cultured EPCs were intravenously transfused, and the transfused EPCs would circulate in the circulatory system. Then, the number of EPCs homing to the neointima of stent‐treated aneurysm may be lower than that of locally administered EPCs. Therefore, the transplanted EPCs may exert weaker effects than endogenous circulating EPCs because only a little ratio of transplanted EPCs would reside in the stent‐treated aneurysm during the healing process.
Differentiation of transplanted BM‐derived EPCs in vivo remains unclear, which is quite different from the situation in vitro. EPCs are highly plastic and can give rise to pro‐angiogenic smooth muscle‐like progeny in vivo 31. It has been reported that transforming growth factor‐β1 induced the transdifferentiation of BM‐derived EPCs toward SMC lineage mediated by transforming growth factor‐β receptor II 32, 33. Therefore, our data indicated that transplanted EPCs probably differentiate into SMCs in the early 2 weeks and into ECs in the late 2 weeks.
There are some limitations of the study. The differentiated double‐positive cells within the neointima were not specifically identified. Furthermore, the labeling technique cannot tolerate long time tracking. In addition, study designed with more time points and large samples would be beneficial to explicitly validate the contribution of transplanted EPCs.
Conclusions
Our study demonstrated that BM‐derived transplanted EPCs contribute to neointima formation and reendothelialization of the elastase‐induced aneurysms after FD treatment. The transplanted EPCs were distributed differently and probably differentiated into different cell types according to the stages of vascular repair in vivo.
Funding
This study was mainly supported by grants from the National Natural Science Foundation of China (No. 81000495, No. 81171093 and No. 81000494) and partially supported from grants (No. 30901556 and No. 81271271).
Conflict of Interests
The authors declare no conflict of interest.
Acknowledgments
Zhen Li and Kai‐Jun Zhao provided assistance with endovascular stenting for flow diverter implantation.
The first three authors contributed equally to this work.
References
- 1. van Gijn J, Kerr RS, Rinkel GJ. Subarachnoid haemorrhage. Lancet 2007;369:306–318. [DOI] [PubMed] [Google Scholar]
- 2. Krings T, Mandell DM, Kiehl TR, et al. Intracranial aneurysms: From vessel wall pathology to therapeutic approach. Nat Rev Neurol 2011;7:547–559. [DOI] [PubMed] [Google Scholar]
- 3. Molyneux AJ, Kerr RS, Birks J, et al. Risk of recurrent subarachnoid haemorrhage, death, or dependence and standardised mortality ratios after clipping or coiling of an intracranial aneurysm in the International Subarachnoid Aneurysm Trial (ISAT): Long‐term follow‐up. Lancet Neurol 2009;8:427–433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Pierot L. Flow diverter stents in the treatment of intracranial aneurysms: Where are we? J Neuroradiol 2011;38:40–46. [DOI] [PubMed] [Google Scholar]
- 5. Asahara T, Murohara T, Sullivan A, et al. Isolation of putative progenitor endothelial cells for angiogenesis. Science 1997;275:964–967. [DOI] [PubMed] [Google Scholar]
- 6. Miller‐Kasprzak E, Jagodzinski PP. Endothelial progenitor cells as a new agent contributing to vascular repair. Arch Immunol Ther Exp (Warsz) 2007;55:247–259. [DOI] [PubMed] [Google Scholar]
- 7. Urbich C, Dimmeler S. Endothelial progenitor cells: Characterization and role in vascular biology. Circ Res 2004;95:343–353. [DOI] [PubMed] [Google Scholar]
- 8. Fang X, Zhao R, Wang K, et al. Bone marrow‐derived endothelial progenitor cells are involved in aneurysm repair in rabbits. J Clin Neurosci 2012;19:1283–1286. [DOI] [PubMed] [Google Scholar]
- 9. Wang K, Huang Q, Hong B, et al. Neck injury is critical to elastase‐induced aneurysm model. AJNR Am J Neuroradiol 2009;30:1685–1687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Carmeliet P, Moons L, Stassen JM, et al. Vascular wound healing and neointima formation induced by perivascular electric injury in mice. Am J Pathol 1997;150:761–776. [PMC free article] [PubMed] [Google Scholar]
- 11. Lin HH, Chen YH, Yet SF, Chau LY. After vascular injury, heme oxygenase‐1/carbon monoxide enhances re‐endothelialization via promoting mobilization of circulating endothelial progenitor cells. J Thromb Haemost 2009;7:1401–1408. [DOI] [PubMed] [Google Scholar]
- 12. Shiba Y, Takahashi M, Yoshioka T, et al. M‐CSF accelerates neointimal formation in the early phase after vascular injury in mice: The critical role of the SDF‐1‐CXCR4 system. Arterioscler Thromb Vasc Biol 2007;27:283–289. [DOI] [PubMed] [Google Scholar]
- 13. Werner N, Priller J, Laufs U, et al. Bone marrow‐derived progenitor cells modulate vascular reendothelialization and neointimal formation: Effect of 3‐hydroxy‐3‐methylglutaryl coenzyme a reductase inhibition. Arterioscler Thromb Vasc Biol 2002;22:1567–1572. [DOI] [PubMed] [Google Scholar]
- 14. Walter DH, Rittig K, Bahlmann FH, et al. Statin therapy accelerates reendothelialization: A novel effect involving mobilization and incorporation of bone marrow‐derived endothelial progenitor cells. Circulation 2002;105:3017–3024. [DOI] [PubMed] [Google Scholar]
- 15. Hibbert B, Ma X, Pourdjabbar A, et al. Inhibition of endothelial progenitor cell glycogen synthase kinase‐3beta results in attenuated neointima formation and enhanced re‐endothelialization after arterial injury. Cardiovasc Res 2009;83:16–23. [DOI] [PubMed] [Google Scholar]
- 16. Sainz J, Sata M. When endothelial progenitor cell says I2 shall limit neointima formation!. Arterioscler Thromb Vasc Biol 2010;30:457–458. [DOI] [PubMed] [Google Scholar]
- 17. Hill JM, Zalos G, Halcox JP, et al. Circulating endothelial progenitor cells, vascular function, and cardiovascular risk. N Engl J Med 2003;348:593–600. [DOI] [PubMed] [Google Scholar]
- 18. Hoh BL, Velat GJ, Wilmer EN, Hosaka K, Fisher RC, Scott EW. A novel murine elastase saccular aneurysm model for studying bone marrow progenitor‐derived cell‐mediated processes in aneurysm formation. Neurosurgery 2010;66:544–550. discussion 550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Griese DP, Ehsan A, Melo LG, et al. Isolation and transplantation of autologous circulating endothelial cells into denuded vessels and prosthetic grafts: Implications for cell‐based vascular therapy. Circulation 2003;108:2710–2715. [DOI] [PubMed] [Google Scholar]
- 20. Fujiyama S, Amano K, Uehira K, et al. Bone marrow monocyte lineage cells adhere on injured endothelium in a monocyte chemoattractant protein‐1‐dependent manner and accelerate reendothelialization as endothelial progenitor cells. Circ Res 2003;93:980–989. [DOI] [PubMed] [Google Scholar]
- 21. Yoshioka T, Takahashi M, Shiba Y, et al. Granulocyte colony‐stimulating factor (G‐CSF) accelerates reendothelialization and reduces neointimal formation after vascular injury in mice. Cardiovasc Res 2006;70:61–69. [DOI] [PubMed] [Google Scholar]
- 22. Takamiya M, Okigaki M, Jin D, et al. Granulocyte colony‐stimulating factor‐mobilized circulating c‐Kit+/Flk‐1 + progenitor cells regenerate endothelium and inhibit neointimal hyperplasia after vascular injury. Arterioscler Thromb Vasc Biol 2006;26:751–757. [DOI] [PubMed] [Google Scholar]
- 23. Tsuzuki M. Bone marrow‐derived cells are not involved in reendothelialized endothelium as endothelial cells after simple endothelial denudation in mice. Basic Res Cardiol 2009;104:601–611. [DOI] [PubMed] [Google Scholar]
- 24. Perry TE, Song M, Despres DJ, et al. Bone marrow‐derived cells do not repair endothelium in a mouse model of chronic endothelial cell dysfunction. Cardiovasc Res 2009;84:317–325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Hagensen MK, Raarup MK, Mortensen MB, et al. Circulating endothelial progenitor cells do not contribute to regeneration of endothelium after murine arterial injury. Cardiovasc Res 2012;93:223–231. [DOI] [PubMed] [Google Scholar]
- 26. Zampetaki A, Kirton JP, Xu Q. Vascular repair by endothelial progenitor cells. Cardiovasc Res 2008;78:413–421. [DOI] [PubMed] [Google Scholar]
- 27. Hu Y, Xu Q. Adventitial biology: Differentiation and function. Arterioscler Thromb Vasc Biol 2011;31:1523–1529. [DOI] [PubMed] [Google Scholar]
- 28. Daniel JM, Bielenberg W, Stieger P, Weinert S, Tillmanns H, Sedding DG. Time‐course analysis on the differentiation of bone marrow‐derived progenitor cells into smooth muscle cells during neointima formation. Arterioscler Thromb Vasc Biol 2010;30:1890–1896. [DOI] [PubMed] [Google Scholar]
- 29. Quah BJ, Warren HS, Parish CR. Monitoring lymphocyte proliferation in vitro and in vivo with the intracellular fluorescent dye carboxyfluorescein diacetate succinimidyl ester. Nat Protoc 2007;2:2049–2056. [DOI] [PubMed] [Google Scholar]
- 30. He T, Smith LA, Harrington S, Nath KA, Caplice NM, Katusic ZS. Transplantation of circulating endothelial progenitor cells restores endothelial function of denuded rabbit carotid arteries. Stroke 2004;35:2378–2384. [DOI] [PubMed] [Google Scholar]
- 31. Moonen JR, Krenning G, Brinker MG, Koerts JA, van Luyn MJ, Harmsen MC. Endothelial progenitor cells give rise to pro‐angiogenic smooth muscle‐like progeny. Cardiovasc Res 2010;86:506–515. [DOI] [PubMed] [Google Scholar]
- 32. Imamura H, Ohta T, Tsunetoshi K, et al. Transdifferentiation of bone marrow‐derived endothelial progenitor cells into the smooth muscle cell lineage mediated by tansforming growth factor‐beta1. Atherosclerosis 2010;211:114–121. [DOI] [PubMed] [Google Scholar]
- 33. Fadini GP, Tjwa M. A role for TGF‐beta in transforming endothelial progenitor cells into neointimal smooth muscle cells. Atherosclerosis 2010;211:32–35. [DOI] [PubMed] [Google Scholar]


