Summary
Aims
To identify long‐term sensorimotor and cognitive deficits and to evaluate structural alterations in brain ischemic mice.
Methods
C57Bl/6J male mice were subjected to 30 min transient middle cerebral artery occlusion (tMCAo) or sham surgery. Sensorimotor deficits, exploratory behavior, and cognitive functions were evaluated up to 6 months. Cortical and subcortical damage were analyzed by MRI multiparameter analysis and histopathology.
Results
tMCAo mice showed significant sensorimotor deficits in the rotarod, negative geotaxis, neuroscore, and beam walk tests. They also showed impairment in exploratory behavior in the open field test and in spatial learning in the Morris water maze. T2‐weighted MRI revealed a volume reduction in injured brain areas at 12 and 24 weeks postinjury. Brain atrophy was shown by MRI and conventional postmortem analysis. Diffusion tensor imaging on the external capsule showed increased values of axial and radial diffusivity. Fiber tracking revealed a reduction in the number and length of ipsilateral fibers.
Conclusions
tMCAo in mice induces sensorimotor and cognitive impairments detectable at least up to 6 months postinjury, associated with brain atrophy, and axonal and myelin damage of the external capsule. These behavioral tests and anatomical investigations may represent important tools in translational studies in cerebral ischemia.
Keywords: Behavior, Brain damage, Brain ischemia, Diffusion tensor imaging, Mice
Introduction
The availability of reliable animal models of focal cerebral ischemia has allowed significant advances in the understanding of the pathophysiology of stroke at cellular and molecular levels and has led to the identification of therapeutical targets 1. However, although several pharmacological strategies have been successful in experimental models, they have all failed in clinical trials, underlying the need for a better translation from experimental to clinical studies 2. Major limitations in many available studies include evaluation of effectiveness mainly or exclusively based on infarct size and lack of functional outcome at clinically relevant time points, that is, weeks or months instead of hours or days after stroke 3, 4, 5. Actually, in patients, cerebral vascular accidents induce brain lesions associated with sensory, motor, and cognitive deficits that progress over time. In addition, functional recovery is a major endpoint in clinical trials 6. Thus besides, acute pathophysiological derangements of clinical stroke, animal models must carefully consider behavioral correlates and long‐term outcome to more closely approximate the human disease condition.
While available data demonstrate behavioral impairment in rats up to 3 months postinjury 7, 8, little is known about the long‐term functional consequences of focal ischemia in mice, which are now the preferred rodent model due to the availability of transgenic and knockout strains. Available studies in ischemic mice explore functional deficits within the first 2 months 9, 10. Including longer time points of functional assessment in experimental stroke, studies becomes even more relevant with the recent interest in neurorestorative strategies, which require a longer window of observation than neuroprotective approaches.
An additional key point is the need to gain insight on the neuroanatomical substrates associated with dysfunction and recovery. Our current understanding of the determinants of functional outcome indicates that next to focal pathology (infarct size), axonal damage and reorganization are primary contributors to behavioral deficits and recovery. Recent neuroanatomical studies in patients with stroke show that the amount of corticospinal tract injury is related to chronic motor dysfunctions 11. MRI now offers excellent anatomical resolution in mice, furthermore diffusion tensor imaging (DTI) allows to assess the integrity and organization of white matter tracts and axonal and myelin damages. Unfortunately, in the experimental stroke setting, studies exploiting the potentiality of this approach are still scarce 12, 13, 14.
This study provides previously unavailable information on the consequences of the ischemic injury on neurological functions and on structural brain damage up to 6 months postinjury. No gold standard exists for behavioral test selection in mice. Tests should complement each other for assessment of global neurological status, sensorimotor coordination/balance, and cognitive impairment 15, 16. We explored an array of complementary tests to reveal both short‐ and long‐term sensorimotor and cognitive deficits induced by 30‐min transient right middle cerebral artery occlusion (tMCAo) in C57Bl/6J male mice and provide a tool to improve long‐term assessment of therapeutic interventions. In addition, we used long‐term MRI multiparameter analysis (3–6 months after injury) along with classical histopathology to assess the degree and progression of cortical and subcortical structural damage and white matter disorganization and relate them to behavioral impairment.
Materials and Methods
Animals
Male C57BL/6J mice (26–28 g, 11 weeks of age, Harlan Laboratories, Italy) were used. Additional information can be found in Supporting Information.
Experimental Design
Stress/anxiety related to manipulation and test exposure can affect behavioral performance in mice. Thus, we minimized the number of tests per mouse defining two study groups as shown in Figure 1. The first included 36 and the second 32 mice (Figure 1, black and white squares, respectively) equally distributed to tMCAo or sham surgery. Ischemia related mortality was 14.7% (2 mice in the first and 3 in the second group). Two additional parameters were obtained in all mice, namely: body weight (a measure of the general well‐being of the mice, Figure 1B) and neuroscore (a sensorimotor test well characterized in our laboratory 17, 18, Figure 2A) also indicated that the two study groups were similar (see results section). Thus, the first group was tested for neuroscore, rotarod, locomotor activity, Morris water maze (all sham = 18, tMCAo = 16), open field (sham = 8, tMCAo = 8) and sacrificed at 12 weeks postsurgery for histological analysis (tMCAo = 16). The second group was tested for neuroscore, negative geotaxis, beam walk, Morris water maze (all sham = 16, tMCAo = 13), open field (sham = 8, tMCAo = 8) and was subjected to multiparameter MRI (sham = 6, tMCAo = 6) and sacrificed at 24 weeks postsurgery for histological analysis (tMCAo = 13) (Figure 1). Details on mice allocation and behavioral protocols are provided in supporting information and supplement table 1. All behavioral tests and MRI analysis were performed by investigators blinded to the experimental conditions.
Figure 1.
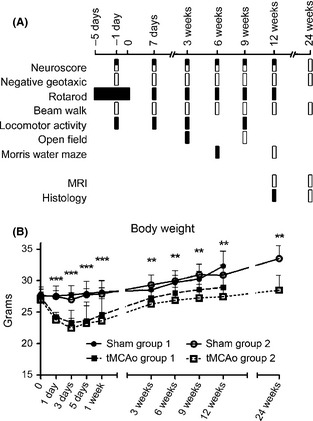
Experimental design. Mice were subjected to two separate study groups (A, black and white squares). Day 0 refers to surgery day. Mice were trained on the accelerated rotarod daily from day −5 to day 0 to generate stable baseline values. Baseline values before surgery were obtained also for neuroscore, negative geotaxis, and beam walk on day −1. All the other tests were performed after surgery. tMCAo mice showed a significant loss of body weight (B) compared with sham‐operated mice at all time points considered. Body weight in the two independent groups of mice (represented with white and black boxes) was never different confirming a comparable degree of injury. Two‐way ANOVA with RM followed by post hoc Tukey's test: **P < 0.01, ***P < 0.001 tMCAo compared with sham‐operated mice.
Figure 2.
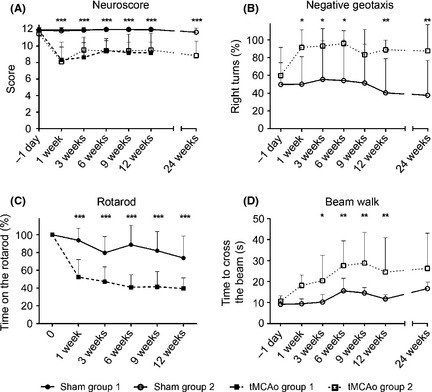
Sensorimotor deficits. Sensorimotor deficits measured by neuroscore (A) negative geotaxis (B), rotarod (C), and beam walk (D) tests. tMCAo mice showed clear motor dysfunction compared with sham‐operated mice for the whole duration of the study in all the tests. Neuroscore in the two independent groups of mice were never different confirming a comparable degree of injury. Data are expressed as mean ± SD. Two‐way ANOVA with RM followed by post hoc Tukey's test: *P < 0.05, **P < 0.01, ***P < 0.001 tMCAo compared to sham‐operated mice.
Surgery
Transient ischemia was achieved by tMCAo by means of a siliconized filament (7–0, Doccol Corp.) introduced into the right internal carotid artery and advanced to block the MCA for 30 min 19. At the end of the ischemic period, the filament was withdrawn and reperfusion allowed. Sham‐operated animals received identical anesthesia (isoflurane 3% followed by 1–1.5% for induction and maintenance, respectively, in a 70%N2O–30%O2 mixture) and isolation of right carotid artery without artery occlusion.
Assessment of Sensorimotor Deficits
Neuroscore
Sensorimotor deficits were assessed by neuroscore test as shown previously 17. Mice were scored from four (normal) to zero (severely impaired) for each of the following indices: (1) forelimb function during walking on the grid and flexion function response during suspension by the tail; (2) hindlimb function during walking on the grid and extension function during suspension by the tail; and (3) resistance to lateral right and left push. The maximum score per animal was 12. Additional details can be found in on‐line supplement materials.
Negative Geotaxis
Bilateral asymmetry was evaluated by negative geotaxis test 20. Mice were placed in a standard position, head downward, on an elevated 45°C inclined wooden board (22 × 40 cm) to turn 180°C and crawl up the board. When leaving the standard position, any leftward or rightward rotation was recorded. Six trials a day were performed, and the percentage of right turns [right/(right + left)] was calculated. In healthy mice, there is a 50% chance that mice make a right turn.
Accelerated Rotarod
Motor coordination and balance was assessed by rotarod test 20. Mice were positioned on the rotating rod, which was then accelerated at a constant rate of 0.12 r.p.m./second from 4 to 40 r.p.m. over 5 min. Mice were trained for 5 days (three consecutive trials/day, intertrial interval: 10 min). The test (three trials, intertrial interval: 10 min) was delivered before surgery (baseline) and at specific times after ischemia, the latency to fall was automatically recorded. Two consecutive passive rotations without walking, but accompanying the rod, were considered as a fall. The average of the three trials was calculated. Data are expressed as percentage of the baseline value.
Beam Walk
Motor coordination and balance was evaluated by beam walk test. After three habituation trials (where the mouse was allowed to walk on the elevated and narrow wooden beam, 5 mm wide and 100 cm in length), mice were tested (3 trials, intertrial interval: 5 min) at specific times. The time spent to cross the beam 21 and the number of foot faults 17 were recorded. The average of the three trials was calculated. Data are expressed as foot faults and mean crossing time.
Assessment of Exploratory and Cognitive Behavior
Locomotor Activity
Locomotor activity was assessed by automated cages with photobeam detectors. Mice were tested in four chambers (TSE Systems, Bad Homburg, Germany) made of gray Plexiglas (40 × 20 × 15 cm) with a stainless steel grid floor and lit with three 1.2 W lamps. The distance travelled (m) by mice inside the chambers was detected by 26 horizontal photobeams along the long axis, 1 cm above the floor and 1.4 cm apart. Locomotor activity was evaluated by TSE software for 60 min 22.
Open Field
This test evaluates exploratory behavior 23, 24. As novelty reactivity greatly influences rodent exploratory activity, each mouse underwent this test only once. The open field consists of a gray Perspex square arena surrounded by walls (40 × 40 × 30 cm) with the floor divided into 25 squares (8 × 8 cm). The nine central squares (24 × 24 cm) represent the “central area” and the surrounding border zone the “outer squares”. The open field contains four different objects fixed in the middle of the four corner squares of the central area. Mice were tested under dim illumination provided by a 60 W lamp placed 1 m above the apparatus and pointed toward the ceiling. Each mouse was placed in the center of the open field, and its behavior was video recorded by Ethovision XT, 5.0 (Noldus Information Technology, Wageningen, The Netherlands) during 5 min. The number of squares crossed (defined as a mouse entering a new square with all four feet), the time spent in the central area of the open field, the number of rearings, and the time spent in contact with the objects (nose toward the object at a minimal distance of 1 cm) were scored later by two operators unaware of the type of surgery mice had received.
Morris Water Maze
The learning and memory performance was assessed in the Morris water maze. A circular pool (100 cm diameter) filled with water (18–20°C) made opaque by a nontoxic white paint and a fixed submerged platform (1 cm below the water surface) were used as reported previously 17. The learning task consisted of 8 trials/day for three consecutive days. Latencies to reach and climb onto the platform were recorded for each trial. Cognitive performance was obtained by averaging the latencies of 24 trials over 3 days. Five days after the learning task, mice were tested for their ability to remember the location of the submerged platform. Animals were allowed to swim for 60 seconds with the platform removed, and their swim paths were video recorded by Ethovision XT, 5.0. A memory score was calculated to grade memory retention as shown previously 17.
Histological Analysis
Atrophic volumes were calculated on 20 μm coronal brain cryosections stained with cresyl violet according to the following formula:
where CA is the area of the contralateral hemisphere, IA is the area of the ipsilateral hemisphere, and d is the distance from a given section (k) to the subsequent section (k+1) 17. Images were acquired on a computer using the image analyzer Analytical Image System (Imaging Research Inc, Brock University, St Catharines, Ontario, Canada), and atrophy was calculated. Histological sections were stained with luxol fast blue standard protocol for qualitative analysis of white matter fiber networks. Details on protocol can be found in online Supplement Materials.
MRI Measurements
Imaging was performed on a 7T small‐bore animal Scanner (Bruker Biospec). The morphological images were obtained with a RARE T2‐weighted sequence that covered the whole mice brain volume. DT‐MRI data were acquired in coronal slices (1 mm thick), using a DT‐EPI sequence.
Volumetric Measurements
The volume measurements of structural MRI images were obtained manually using custom made software as previously described 25. As extensive atrophy associated with ischemia prevented an automated selection based on registration from an anatomical image, for each animal, the ROI was manually chosen and drawn on the images for volumetric assessment (Figure 4). We computed the lesion volume including the whole infarcted tissue across different brain areas. This measurement did not include the ventricle volume. Measurements of brain atrophy in whole brain, cortex, hippocampus, and caudate–putamen 26 included residual T2w hypointense tissue. Data from each animal were obtained by the integration of averaged ROI area for slice thickness.
Figure 4.
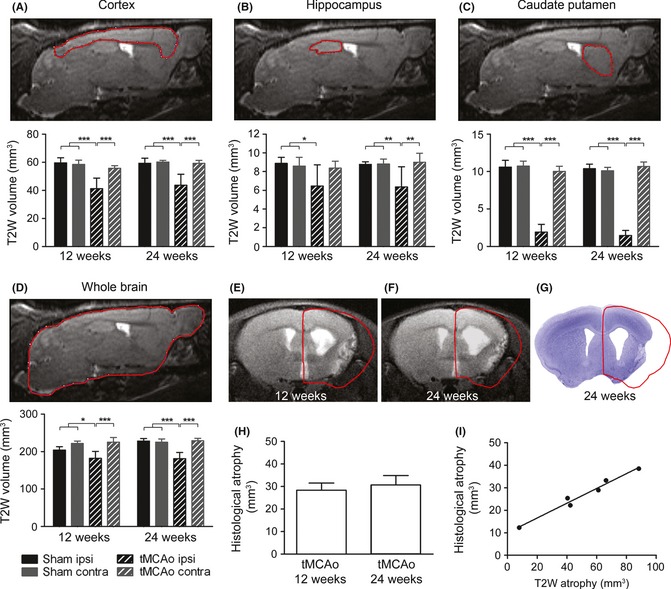
Structural damage analyzed by MRI and conventional histology. Representative images depicting the ROI considered for analysis of structural damage by MRI in cortex, hippocampus, caudate‐putamen, and whole brain (A–D, upper panels) and their quantitative evaluation (lower panels) showing that tMCAo produced a significant volume reduction at 12 and 24 weeks postinjury in the injured cortex, hippocampus, caudate–putamen compared with the corresponding area in sham‐operated mice. Representative coronal section showing the extensive atrophy of the ipsilateral hemisphere by MRI at 12 and 24 weeks (E, F) and by conventional histology at 24 weeks in the same mouse (G). Whole brain atrophy quantified by conventional histology shows comparable degree of atrophy at 12 and 24 weeks after tMCAo (H). Whole brain atrophy quantified by MRI strongly correlated with that measured by conventional histology (I, correlation Pearson r:0.99, P < 0.01, F). Data are expressed as mean ± SD. Two‐way ANOVA with RM and post hoc Tukey's test: P < 0.01. *P < 0.05, **P < 0.01, ***P < 0.001.
Diffusion Tensor Measurements
The diffusion tensor and the fiber tracking were computed using the freely available software MedINRIA (http://med.inria.fr/). The external capsule was analyzed. Extensive atrophy associated with ischemia prevented an automated selection based on registration from an anatomical image, thus the ROI (area ~0.18 mm2) was manually chosen on ipsilateral and contralateral external capsule from bregma ~ −0.38 mm to bregma ~ −1.46 mm. To minimize bias related to manual selection, the ROI was positioned at ~0.42 mm from the cingulum (~0.43 ± 0.06 mm ipsilateral and ~0.41 ± 0.07 mm contralateral for sham‐operated mice) for quantitative comparison of the DT‐MRI values (See Figure 6 A,B). We computed the fractional anisotropy (FA) and axial (λ//) and radial diffusivity (λ┴).
Figure 6.
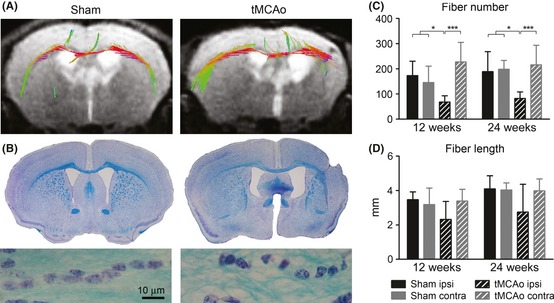
Tracking of fibers passing in external capsule. Representative fiber tracking in the external capsule in a sham‐operated or tMCAo brain (A) and microphotographs depicting altered orientation of external capsule fiber bundles stained by Luxol fast blue (B). Quantification of number (C) and length (D) of fibers at 12 and 24 weeks postsurgery showing a significant reduction in the number of ipsilateral fibers and in mean fiber length in injured compared to sham‐operated mice. Two‐way ANOVA with RM and post hoc Tukey's test: *P < 0.05, ***P < 0.001.
We determined an index of localized alterations in the white matter on the fiber network. On two consecutive slices, square regions of interest (~0.18 mm2 area) were positioned on the external capsule, both for the contralateral and ipsilateral hemisphere. We obtained two different indices from this analysis: the measurement of fiber mean length and the fiber number 13. Detailed information on MRI measurements can be found in online Supporting Informations.
Statistical Methods
For statistical analysis, we used standard software packages (GraphPad Prism version 4.00, La Jolla, CA, USA or JMP, version 7.0). All data are presented as mean and SD. For changes in body weight, neuroscore, negative geotaxis, rotarod, beam walk, distance travelled, learning latencies, volumetric measurements, and DTI data, the comparison between groups was performed using two‐way mixed‐factorial ANOVA with surgery as between‐subjects factor and weeks as within‐subjects factor. The open field and the memory score were analyzed by the two‐way ANOVA (with surgery and weeks as main factors). The degrees of freedom for f‐test were indicated in brackets (treatment, residual). Post hoc analysis was carried out by Tukey test.
Results
Two groups of tMCAo and sham‐operated mice were evaluated for behavioral and anatomical outcome at different times postinjury as indicated in Figure 1.
Body Weight
Changes in mice body weight are shown in Figure 1B. tMCAo induced a significant loss of body weight compared with sham‐operated mice at all time points considered (Figure 1B, Fsurgery(3,52) = 24.3, P < 0.01; Fweeks(8,416) = 105.6, P < 0.01; Fint(24,416) = 6.04 P < 0.01). As expected, body weight changes between the two groups of tMCAo mice did not show a statistically significant difference confirming the consistency of the procedure.
Sensorimotor Deficits
The neuroscore evidenced a significant difference between tMCAo and sham‐operated mice at all time points considered (Figure 2A, Fsurgery(3,52) = 38.3, P < 0.01; Fweeks(5,260) = 33.09, P < 0.01; Fint(15,260) = 38.3 P < 0.01). The test was performed on each of the two groups of mice (Figure 2A) who showed a similar degree of sensorimotor dysfunction indicating once again a similar degree of injury. Thus, having shown that body weight and neuroscore were similar, from here on the two groups of mice were considered equal. The negative geotaxis test revealed a significant increase in right turns from 1 up to 24 weeks after surgery in tMCAo compared with sham‐operated mice (Figure 2B, Fsurgery(1,22) = 25.4, P < 0.01; Fweeks(8,176) = 2.48, P < 0.05; Fint(8,176) = 2.12, P < 0.05). Similarly, the rotarod showed a significant reduction in time spent on the rod in tMCAo mice from 1 up to 12 weeks postinjury (Figure 2C, Fsurgery(1,32) = 63.1, P < 0.01; Fweeks(5,160) = 48.94, P < 0.01; Fint(5,160) = 14.26, P < 0.01). Longer times were not assessed given the evidence that age and weight gain may affect the performance 27. Finally, in the beam walk test, a significantly longer time to cross the beam was evident in tMCAo mice between 3 and 12 weeks postinjury compared with sham‐operated mice (Figure 2D, Fsurgery(1,22) = 13.02, P < 0.01; Fweeks(8,132) = 11.48, P < 0.01; Fint(8,132) = 2.4, P < 0.01). No significant impairment could be detected when assessing the number of foot faults (not shown).
Exploratory and Cognitive Behavior
Two‐way ANOVA of distance traveled in the automated cage during the total observation period found no significant effect of injury (F(1,16) = 0.2, n.s.) on locomotor activity, but significant effect of weeks (F(3,48) = 84.5, P < 0.01) with no interaction between injury and weeks (F(3,48) = 1.3, n.s.). A clear reduction in mice locomotor activity was evident after surgery in both tMCAo and sham‐operated mice (Figure 3A). The open field test revealed a significant impairment in exploratory behavior, measured as time spent in contact with objects in tMCAo mice at both 3 and 9 weeks postinjury (Figure 3B, Fsurgery(1,30) = 7.8; P < 0.01; Fweeks(1,30) = 0.01, n.s.; Fint(1,30) = 0.3, n.s.). No significant effect of injury was found on time spent in the center of the open field, number of inner squares crossed, total squares crossed (inner + outer), number of rearings at each of the time points evaluated (data not shown).
Figure 3.
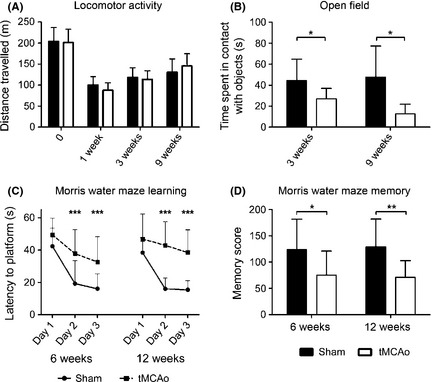
Exploratory and cognitive behavior. A clear reduction in locomotor activity was evident after surgery in both tMCAo and sham‐operated mice with no injury effect (A). A significant effect of tMCAo on exploratory behavior evaluated by open field test as time spent in contact with objects was evident at both 3 and 9 (B) weeks. Learning behavior was assessed as latency to locate the hidden platform in the Morris water maze test at 6 or 12 weeks after surgery (C). A significant longer time to platform was evident on day 2 and 3 of training in tMCAo compared with sham‐operated mice. Five days after the end of the learning test, tMCAo mice showed a memory retention deficit both at 6 and 12 weeks after injury (D). Data are expressed as mean ± SD. Two‐way ANOVA (A–D) and post hoc Tukey's test: *P < 0.05, **P < 0.01, ***P < 0.001 tMCAo compared with sham‐operated mice.
Six or 12 weeks after tMCAo, 2 independent groups of mice were evaluated for their ability to learn the position of a hidden platform in the Morris water maze. All animals were able to swim without visible alterations in swimming ability (swimming speed at 6 weeks: tMCAo 18.9 ± 4.1, sham‐operated 18.7 ± 2.4; 12 weeks: tMCAo18.8 ± 3.9, sham‐operated 18.5 ± 2.4 cm/second). However, tMCAo mice showed a learning dysfunction at both time points considered (Figure 3C, latency to platform at 6 weeks: Fsurgery(1,32) = 16.2, P < 0.01; Fweeks(2,64) = 49.9, P < 0.01; Fint(2,64) = 3.7, P < 0.05; 12 weeks: Fsurgery(1,22) = 24.3, P < 0.01 Fweeks(2,44) = 26.6, P < 0.01; Fint(2,44) = 9.0, P < 0.01). Moreover, 5 days after learning, tMCAo mice showed a decreased memory score compared with sham‐operated mice at both time points (Figure 3D, Fsurgery(1,52) = 16.3, P < 0.01; Fweeks(1,52) = 0.01, n.s.; Fint(1,52) = 0.07, n.s).
Anatomical Damage: Structural MRI and Conventional Histology
tMCAo produced a significant volume reduction at 12 and 24 weeks postinjury in the injured cortex, hippocampus, caudate–putamen compared with the corresponding area in sham mice, measured by T2‐weighted MRI (T2w, cortex: Fsurgery(1,10) = 26.6, P < 0.01; hippocampus: Fsurgery(1,10) = 7.01, P < 0.05; caudate–putamen: Fsurgery(1,10) = 488.5, P < 0.01, Figure 4A–C). Thus, a global atrophy of the injured hemisphere was detected at 12 and 24 weeks (Fsurgery(1,10) = 25.2, P < 0.01, Figure 4D). Whole brain atrophy was also measured by conventional histology at both time points showing values that strongly correlated with those measured by T2w (correlation Pearson r: 0.99, P < 0.01, Figure 4G–I). A strong correlation was observed between learning and hippocampal T2w damage at 12 and 24 weeks (correlation Pearson r:−0.89, P < 0.05) while no relationship was detected considering the other brain areas nor the whole brain and the other behavioral tests (data not shown). No changes were detected in the contralateral side for all considered parameters.
DTI Analysis
Diffusion tensor imaging data were obtained on the external capsule in order to assess changes in the corticospinal tract in the selected region of interest (ROI, Figure 5A). Increased values of axial and radial diffusivity were found in the external capsule at both time points considered compared with sham‐operated mice with no change in FA (axial diffusivity: Fsurgery(1,11) = 50.0, P < 0.01; Fweeks(1,11) = 2.7, n.s.; Fint(1,11) = 0.1, n.s; radial diffusivity: Fsurgery(1,11) = 36.2, P < 0.01; Fweeks(1,11) = 0 n.s.; Fint(1,11) = 0, n.s. Figure 5B–D). Fiber tracking (Figure 6A) revealed a significant reduction in the number of ipsilateral fibers in injured mice (Fsurgery(1,11) = 28.7, P < 0.01; Fweeks(1,11) = 0.5, n.s.; Fint(1,11) = 0, n.s. Figure 6C) and in mean fiber length (Fsurgery(1,11) = 5.2, P < 0.05; Fweeks(1,11) = 3.7, n.s.; Fint(1,11) = 0.2, n.s. Figure 6D). No changes were detected in the contralateral side for all considered parameters.
Figure 5.
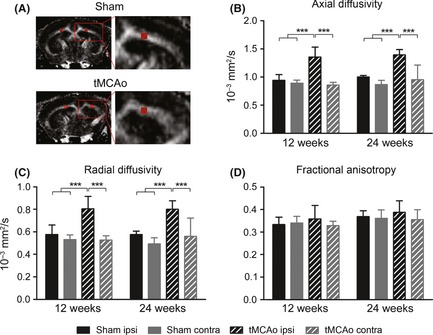
Quantitative analysis of diffusion tensor imaging parameters in the external capsule. Representation of rostrocaudal fractional anisotropy map and ROI selection in the external capsule in sham‐operated and tMCAo (A) mice. Quantification of axial diffusivity (B), radial diffusivity (C), and fractional anisotropy (D) within the ROI showing that axial and radial diffusivity were increased in the external capsule at both time points considered compared to sham‐operated mice with no change in fractional anisotropy. Data are expressed as mean ± SD. Two‐way ANOVA with RM and post hoc Tukey's test: P < 0.01. ***P < 0.001.
A disorganization of fiber network in the external capsule was confirmed by Luxol fast blue staining (Figure 6B).
Discussion
This study shows that tMCAo in mice induces sensorimotor, exploratory and cognitive impairments which are detectable at least up to 6 months postinjury and are associated with atrophy of the injured brain, as well as axonal and myelin damage of the external capsule.
After tMCAo, weight changes are known to reflect the general well‐being of the animal and the degree of brain injury 28. In our hands, 30 min right tMCAo produced a significant and persistent loss of body weight compared with sham‐operated mice indicating a severe brain damage. In this condition, we assessed the presence of chronic sensorimotor deficits that include bilateral asymmetry and motor coordination and balance using negative geotaxis and rotarod tests, respectively. These tests demonstrated a wide and stable difference between tMCAo and sham‐operated mice on motor function over time (over 24 and 12 weeks, respectively) indicating their ability to detect long‐term effects. Negative geotaxis has been used in two different studies up to 1 week postinjury showing variable results. Li et al. 29, showed a slight but significant difference up to 1 week in female C57Bl/6 tMCAo mice. Conversely, Ferrara et al. 20, observed a spontaneous recovery already at 1 week in male 129/Sv tMCAo mice. This strain is known to be relatively hypoactive compared with C57Bl/6 mice 30. Altogether these data suggest that mouse gender and strain should be taken into account for appropriate test selection, and our data support the finding that C57Bl/6 male mice, that are the most commonly used background strain for genetically engineered mice 31, are more responsive to tMCAo than other strains.
Rotarod test has been used in several studies in mice yielding mixed results 27. The majority of available data report only transient motor coordination deficits with a full recovery of function by approximately 1 week post tMCAo 20, 32, 33. Thus, long‐term sensitivity is uncertain and as outlined in a recent review by Balkaya et al., 15, “may depend on testing routine”. In this contest, we believe that the choice of a smooth versus striated rod improves sensitivity by minimizing the mice from being able to cling to the beam 34. According to the literature, striated rod unveils mostly transient motor coordination deficits 20, 33, and while using the smooth rod, we were able to detect a persistent impairment (up to 12w postinjury). Recently, rotarod was used to assess motor learning performance after tMCAo in mice not trained prior to surgery 35. In this setting, the authors showed a comparable performance of tMCAo and sham mice on the first exposure to the task (2 days after surgery), but a significant and persistent injury effect in the following days of testing (up to 8 weeks). Our data obtained in pretrained mice show not only a learning motor impairment but also clear balance and coordination deficits after tMCAo with no signs of improvement in the task over time.
As neuroscore and beam walk show consistent sensorimotor impairments in traumatic brain injury (TBI) 17, 36, we sought to assess whether these tests could detect deficits induced by tMCAo. Both tests were successfully to significantly distinguish sham versus tMCAo mice. We observed, however, a smaller injury effect then reported for TBI mice 17, 36. Thus, their ability to disclose a treatment effect at chronic stages still needs to be verified.
A clear reduction in locomotor activity was evident after surgery in both tMCAo and sham‐operated mice. When analyzing longitudinal data obtained on automated cages, it should be noted that novelty reactivity greatly influences rodent exploratory behavior 37. This could explain why a clear reduction in locomotor activity was evident after surgery in sham mice indicating a habituation to the task. Alternatively, an unspecific surgery effect could be responsible for this decrease, however, as sensorimotor performance was not affected by sham surgery in any of the tests evaluated, this explanation seems unlikely. Three and 9 weeks after tMCAo, spontaneous exploratory function, assessed by the time spent in contact with objects in the open field arena, was impaired. This parameter provides important information on modifications of hyper/hypo activity induced by a selected treatment, as we have shown previously 23. The observation that the other parameters assessed in the open field test were not changed indicates that “anxiety” and motor behavior are not affected in our tMCAo mice.
At 6 and 12 weeks after tMCAo, learning and spatial memory processes were impaired. As regards the assessment of cognitive function after tMCAo, the results in the literature are scarce and often conflicting. Ischemic mice tested in the water maze task may present cognitive deficits lasting 3 weeks postsurgery 38 or more often remain indifferent to ischemia 16, 39, 40. These different results could be explained by the variability in the protocols used (number of trials per day, intertrial interval and delay for the memory score, pre or postsurgical training, severity of injury). Actually, our protocol has successfully evidenced a learning and memory dysfunction, which is measurable and persistent.
The volumetric characterization of ischemic damage on MRI T2w images acquired 12 and 24 weeks after injury showed a clear atrophy of lesioned cortical and subcortical regions leading to a global atrophy that highly correlated with that quantified by conventional histology. Whereas the correlation between acute histological lesions and early behavioral impairment is well documented 16, less is known about the long‐term evolution of this relationship. We observed a selective correlation between learning and hippocampal T2w damage at 12 and 24 weeks (correlation Pearson r: −0.89, P < 0.05), thus confirming the relevant role of hippocampus in spatial learning 41.
This study shows that axial and radial diffusivity indexes that are related to axonal and myelinic damage, respectively, strongly increase in the ipsilateral external capsule at 12 and 24 weeks post‐tMCAo with no change in FA. Brain tissue affected by severe ischemia often progresses to microscopic cavitation at chronic stages 42. This histological feature leads to an increase in water mobility due to the fluid‐filled cystic regions, thus explaining the marked increase in the two diffusivity indexes, in line with what has been found at 8 weeks in rats 12. Furthermore, the apparently surprising normal FA values can be mathematically explained by the equal axial to radial diffusivity ratio (complete formula in method section). Altogether these data suggest that in the chronic phase, normal FA is not always correlated with neurological recovery and that axial and radial diffusivity parameters rather than FA, should be used as indices of ischemic cerebral damage at chronic stages.
We further explored the structure of the fiber bundles in this ROI and found a slight reduction in the fiber mean length associated to a clear decrease in fiber number that was confirmed on histological sections. Similarly, to what reported by Bihel et al. 13 in marmosets, we could find a marked disorganization of the fibers in the external capsule indicating a loss of connectivity clearly visible on tractographs and on histological sections. Thus, our DTI data show that alteration of the axonal and myelinic component of external capsule fiber structure, that is part of the corticospinal tract, may underlie the persistent behavioral deficits detected in this study. We are aware that ischemic damage in one cerebral hemisphere may cause neuroplasticity via axonal regrowth from contralateral hemisphere, thus shadowing the neurobehavioral effects 43. Our DTI analysis, however, showed no signs of contralateral rearrangements. These data coupled with a consolidation of the behavioral impairments at 12 and 24 weeks suggest that in our condition contralateral neuroplasticity is not playing a major role.
Conclusions
This study provides evidence of long‐term sensorimotor and cognitive impairments induced by tMCAo that are associated with atrophy of the injured brain areas, and a clear pattern of changes in axial and radial diffusivity that reflects axonal and myelin damage of the external capsule. The behavioral tests and anatomical approaches identified here represent important tools in translational studies in cerebral ischemia.
Conflict of Interests
The authors declare no conflict of interest.
References
- 1. Iadecola C, Anrather J. Stroke research at a crossroad: Asking the brain for directions. Nat Neurosci 2011;14:1363–1368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Lansberg MG, Bluhmki E, Thijs VN. Efficacy and safety of tissue plasminogen activator 3 to 4.5 hours after acute ischemic stroke: A metaanalysis. Stroke 2009;40:2438–2441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Fisher M, Feuerstein G, Howells DW, et al. Update of the stroke therapy academic industry roundtable preclinical recommendations. Stroke 2009;40:2244–2250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Dirnagl U. Bench to bedside: The quest for quality in experimental stroke research. J Cereb Blood Flow Metab 2006;26:1465–1478. [DOI] [PubMed] [Google Scholar]
- 5. Wechsler L, Steindler D, Borlongan C, et al. Stem Cell Therapies as an Emerging Paradigm in Stroke (STEPS): Bridging basic and clinical science for cellular and neurogenic factor therapy in treating stroke. Stroke 2009;40:510–515. [DOI] [PubMed] [Google Scholar]
- 6. Endres M, Engelhardt B, Koistinaho J, et al. Improving outcome after stroke: Overcoming the translational roadblock. Cerebrovasc Dis 2008;25:268–278. [DOI] [PubMed] [Google Scholar]
- 7. Encarnacion A, Horie N, Keren‐Gill H, Bliss TM, Steinberg GK, Shamloo M. Long‐term behavioral assessment of function in an experimental model for ischemic stroke. J Neurosci Methods 2011;196:247–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Villa P, Van Beek J, Larsen AK, et al. Reduced functional deficits, neuroinflammation, and secondary tissue damage after treatment of stroke by nonerythropoietic erythropoietin derivatives. J Cereb Blood Flow Metab 2007;27:552–563. [DOI] [PubMed] [Google Scholar]
- 9. Bacigaluppi M, Pluchino S, Peruzzotti‐Jametti L, et al. Delayed post‐ischaemic neuroprotection following systemic neural stem cell transplantation involves multiple mechanisms. Brain 2009;132:2239–2251. [DOI] [PubMed] [Google Scholar]
- 10. Reitmeir R, Kilic E, Reinboth BS, et al. Vascular endothelial growth factor induces contralesional corticobulbar plasticity and functional neurological recovery in the ischemic brain. Acta Neuropathol 2012;123:273–284. [DOI] [PubMed] [Google Scholar]
- 11. Abela E, Missimer J, Wiest R, et al. Lesions to primary sensory and posterior parietal cortices impair recovery from hand paresis after stroke. PLoS ONE 2012;7:e31275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Pitkonen M, Abo‐Ramadan U, Marinkovic I, et al. Long‐term evolution of diffusion tensor indices after temporary experimental ischemic stroke in rats. Brain Res 2012;1445:103–110. [DOI] [PubMed] [Google Scholar]
- 13. Bihel E, Roussel S, Toutain J, Bernaudin M, Touzani O. Diffusion tensor MRI reveals chronic alterations in white matter despite the absence of a visible ischemic lesion on conventional MRI: A nonhuman primate study. Stroke 2011;42:1412–1419. [DOI] [PubMed] [Google Scholar]
- 14. Jiang Q, Zhang ZG, Ding GL, et al. MRI detects white matter reorganization after neural progenitor cell treatment of stroke. Neuroimage 2006;32:1080–1089. [DOI] [PubMed] [Google Scholar]
- 15. Balkaya M, Kröber JM, Rex A, Endres M. Assessing post‐stroke behavior in mouse models of focal ischemia. J Cereb Blood Flow Metab 2013;33:330–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Bouët V, Freret T, Toutain J, Divoux D, Boulouard M, Schumann‐Bard P. Sensorimotor and cognitive deficits after transient middle cerebral artery occlusion in the mouse. Exp Neurol 2007;203:555–567. [DOI] [PubMed] [Google Scholar]
- 17. Zanier ER, Montinaro M, Vigano M, et al. Human umbilical cord blood mesenchymal stem cells protect mice brain after trauma. Crit Care Med 2011;39:2501–2510. [DOI] [PubMed] [Google Scholar]
- 18. Longhi L, Perego C, Ortolano F, et al. C1‐inhibitor attenuates neurobehavioral deficits and reduces contusion volume after controlled cortical impact brain injury in mice. Crit Care Med 2009;37:659–665. [DOI] [PubMed] [Google Scholar]
- 19. Gesuete R, Storini C, Fantin A, et al. Recombinant C1 inhibitor in brain ischemic injury. Ann Neurol 2009;66:332–342. [DOI] [PubMed] [Google Scholar]
- 20. Ferrara A, El Bejaoui S, Seyen S, Tirelli E, Plumier J‐C. The usefulness of operant conditioning procedures to assess long‐lasting deficits following transient focal ischemia in mice. Behav Brain Res 2009;205:525–534. [DOI] [PubMed] [Google Scholar]
- 21. Fan Y, Shen F, Frenzel T, et al. Endothelial progenitor cell transplantation improves long‐term stroke outcome in mice. Ann Neurol 2010;67:488–497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Orrù A, Fujani D, Cassina C, Conti M, Di Clemente A, Cervo L. Operant, oral alcoholic beer self‐administration by C57BL/6J mice: Effect of BHF177, a positive allosteric modulator of GABA(B) receptors. Psychopharmacology 2012;222:685–700. [DOI] [PubMed] [Google Scholar]
- 23. Capone C, Frigerio S, Fumagalli S, et al. Neurosphere‐derived cells exert a neuroprotective action by changing the ischemic microenvironment. PLoS ONE 2007;2:e373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Walsh RN, Cummins RA. The Open‐Field Test: A critical review. Psychol Bull 1976;83:482–504. [PubMed] [Google Scholar]
- 25. Cipriani R, Villa P, Chece G, et al. CX3CL1 is neuroprotective in permanent focal cerebral ischemia in rodents. J Neurosci 2011;31:16327–16335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Paxinos G, Franklin KBJ. The Mouse Brain in Stereotaxic Coordinates. Amsterdam; Boston: Elsevier Academic Press, 2004. [Google Scholar]
- 27. Zarruk JG, Garcia‐Yebenes I, Romera VG, et al. Neurological tests for functional outcome assessment in rodent models of ischaemic stroke. Rev Neurol 2011;53:607–618. [PubMed] [Google Scholar]
- 28. Schöller K, Zausinger S, Baethmann A, Schmid‐Elsaesser R. Neuroprotection in ischemic stroke–combination drug therapy and mild hypothermia in a rat model of permanent focal cerebral ischemia. Brain Res 2004;1023:272–278. [DOI] [PubMed] [Google Scholar]
- 29. Li X, Blizzard KK, Zeng Z, DeVries AC, Hurn PD, McCullough LD. Chronic behavioral testing after focal ischemia in the mouse: Functional recovery and the effects of gender. Exp Neurol 2004;187:94–104. [DOI] [PubMed] [Google Scholar]
- 30. Winter B, Juckel G, Viktorov I, et al. Anxious and hyperactive phenotype following brief ischemic episodes in mice. Biol Psychiatry 2005;57:1166–1175. [DOI] [PubMed] [Google Scholar]
- 31. Matsuo N, Takao K, Nakanishi K, Yamasaki N, Tanda K, Miyakawa T. Behavioral profiles of three C57BL/6 substrains. Front Behav Neurosci 2010;4:29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Oyamada N, Sone M, Miyashita K, et al. The role of mineralocorticoid receptor expression in brain remodeling after cerebral ischemia. Endocrinology 2008;149:3764–3777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Bouet V, Boulouard M, Toutain J, et al. The adhesive removal test: A sensitive method to assess sensorimotor deficits in mice. Nat Protoc 2009;4:1560–1564. [DOI] [PubMed] [Google Scholar]
- 34. Rattray I, Smith E, Gale R, Matsumoto K, Bates GP, Modo M. Correlations of Behavioral Deficits with Brain Pathology Assessed through Longitudinal MRI and Histopathology in the R6/2 Mouse Model of HD. PLoS ONE 2013;8:e60012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Truong DT, Venna VR, McCullough LD, Fitch RH. Deficits in auditory, cognitive, and motor processing following reversible middle cerebral artery occlusion in mice. Exp Neurol 2012;238:114–121. [DOI] [PubMed] [Google Scholar]
- 36. Ortolano F, Colombo A, Zanier ER, et al. c‐Jun N‐terminal kinase pathway activation in human and experimental cerebral contusion. J Neuropathol Exp Neurol 2009;68:964–971. [DOI] [PubMed] [Google Scholar]
- 37. Bolivar VJ. Intrasession and intersession habituation in mice: From inbred strain variability to linkage analysis. Neurobiol Learn Mem 2009;92:206–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Gibson CL, Bath PMW, Murphy SP. G‐CSF reduces infarct volume and improves functional outcome after transient focal cerebral ischemia in mice. J Cereb Blood Flow Metab 2005;25:431–439. [DOI] [PubMed] [Google Scholar]
- 39. Klapdor K, Van Der Staay FJ. Repeated acquisition of a spatial navigation task in mice: Effects of spacing of trials and of unilateral middle cerebral artery occlusion. Physiol Behav 1998;63:903–909. [DOI] [PubMed] [Google Scholar]
- 40. Winter B, Bert B, Fink H, Dirnagl U, Endres M. Dysexecutive syndrome after mild cerebral ischemia? Mice learn normally but have deficits in strategy switching. Stroke 2004;35:191–195. [DOI] [PubMed] [Google Scholar]
- 41. D'Hooge R, De Deyn PP. Applications of the Morris water maze in the study of learning and memory. Brain Res Brain Res Rev 2001;36:60–90. [DOI] [PubMed] [Google Scholar]
- 42. Mena H, Cadavid D, Rushing EJ. Human cerebral infarct: A proposed histopathologic classification based on 137 cases. Acta Neuropathol 2004;108:524–530. [DOI] [PubMed] [Google Scholar]
- 43. Nudo RJ. Postinfarct cortical plasticity and behavioral recovery. Stroke 2007;38:840–845. [DOI] [PubMed] [Google Scholar]


