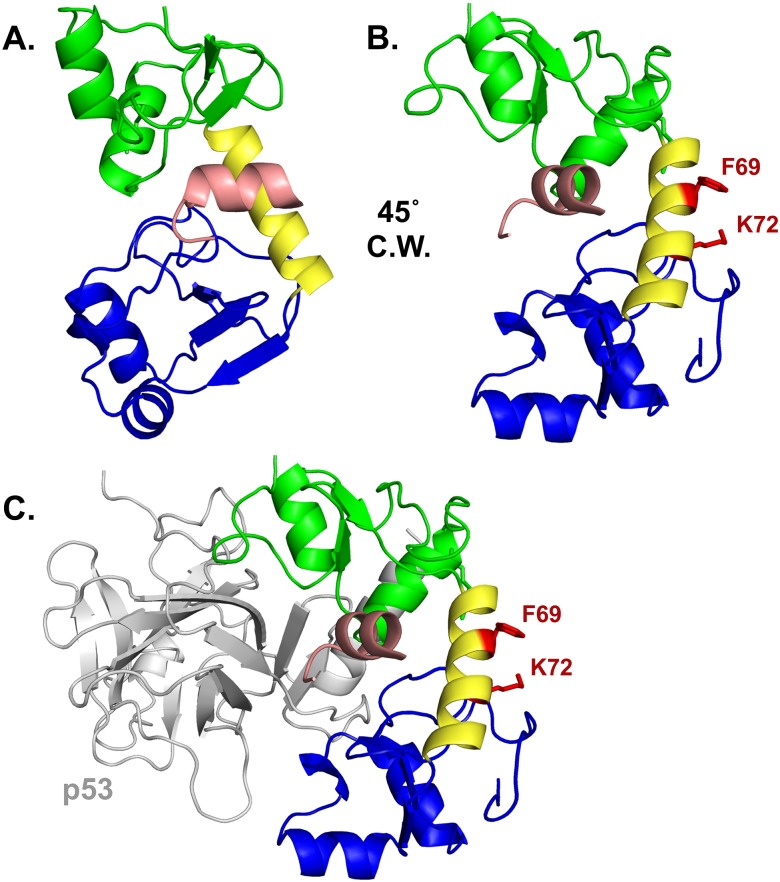
Fig 6. Amino acid side chains F69 and K72 define a novel substrate interaction domain on 16E6.
(A) HPV16 E6 structure (PDB file 4GIZ) showing the amino-terminal zinc-structured domain in green, connecting alpha helix in yellow, and the carboxy-terminal zinc-structured domain in blue. The E6 protein is complexed with the LXXLL peptide of E6AP (pictured in light pink). (B) The E6 protein depicted in A is rotated 45° clockwise (C.W.) and the F69 and K72 residues and their side chains are highlighted in red. (C) A similar view as part B is shown complexed with the core p53 DNA binding domain (grey). The E6 interaction face with p53 is opposite the F69 and K72 residues.