Figure 2.
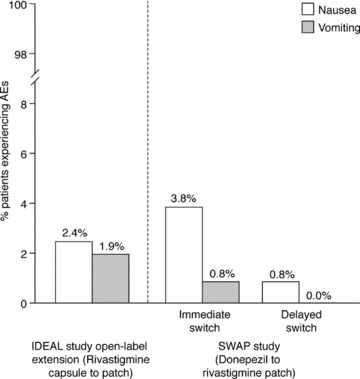
Percentages of patients who experienced the adverse events of nausea and vomiting after switching from rivastigmine capsules to rivastigmine patch (nausea and vomiting reported during the “switch” phase to the 9.5 mg/24 h patch [weeks 1–4 of the open‐label extension] only), and from donepezil tablets to rivastigmine patch (safety populations).
