Abstract.
Many species of Amblyomma ticks are commonly found infesting wild birds in South America, where birds are important hosts for several arboviruses, such as West Nile virus (WNV) and St. Louis encephalitis virus (SLEV). In this study, WNV and SLEV transmission experiments were performed to evaluate the vector competence of three South American tick species: Amblyomma ovale, Amblyomma tigrinum, and Amblyomma tonelliae. Larval and nymphal ticks of each species were allowed to feed on chicks needle inoculated with WNV or SLEV. All three Amblyomma species acquired either WNV or SLEV through larval feeding, with infection rates varying from 3.1% to 100% for WNV and from 0% to 35.7% for SLEV in engorged larvae. Transstadial perpetuation of the viruses was demonstrated in the molted nymphs, with WNV infection rates varying from 0% to 33.7% and SLEV infection rates from 13.6% to 23.8%. Although nymphal ticks also acquired either virus through feeding, transstadial perpetuation to adult ticks was lower, with virus detection in only 3.2% of A. tigrinum and 11.5% of A. tonelliae unfed adult ticks. On the other hand, vector competence for nymphs (exposed to WNV or SLEV through larval feeding) and adult ticks (exposed to WNV or SLEV through larval or nymphal feeding) was null in all cases. Although our results indicate transstadial perpetuation of WNV or SLEV in the three tick species, the ticks were not competent to transmit these agents to susceptible hosts. The role of these ixodid tick species in the epidemiology of WNV and SLEV might be insignificant, even though at least A. ovale and A. tigrinum are frequent bird ticks in Latin America, so the virus could survive winter in the fed larvae. However, future studies are required to determine the implications that this could have, as well as analyze the vector competence of other common bird tick species in South America.
INTRODUCTION
West Nile virus (WNV) and St. Louis encephalitis virus (SLEV) are emerging arboviruses (arthropod-borne viruses). Both are members of the Japanese encephalitis serogroup within the genus Flavivirus (family Flaviviridae) whose distributions did not overlap before the invasion of North America by WNV in 1999.1,2 West Nile virus was isolated for the first time in 1937 from the blood of a febrile woman in the West Nile district in Uganda.3 West Nile virus has propagated to a vast region of the globe and it is now considered one of the most important causative agent of viral encephalitis.4 Presently, it has been found in Africa, the Middle East, parts of Europe, Southern Asia, Australia, and America.5 In the American continent, WNV emerged in the United States as an important medical and veterinary pathogen.1 The first activity report in the southern cone of South America was in April 2006, when three horses died because of WNV in Argentina.6 However, established transmission foci in Argentina were detected by 2005, as evidenced by resident birds that tested serologically positive for WNV (Cardinalidae, Columbidae, Falconidae, Furnariidae, Icteridae, and Turdidae, among other bird families).7 St. Louis encephalitis virus was isolated for the first time in 1933 during a human encephalitis outbreak in St. Louis, MO, and, currently it is exclusively distributed in the American continent.8 In Argentina, the last diagnosed human case by SLEV was in 1987 when a febrile human was reported in Buenos Aires city.9 Seventeen years later, SLEV reemerged in the central region during 2002.10 Since then, outbreaks have been reported in the provinces of Córdoba (2005),11 Entre Rios (2006), Buenos Aires (2010), and San Juan (2011).12
The multi-host–vector profile of these viruses gives them the ability to invade and colonize diverse ecosystems.13 In Argentina, both viruses are widely distributed, encompassing tropical, subtropical, and semidesert ecosystems.7 This ecological plasticity indicates the presence of alternative transmission mechanisms allowing their maintenance in different situations not compatible with mosquito vector transmission. These potential mechanisms include annual introductions by migratory birds, viral persistence in nondiapausing mosquitoes, or alternative arthropod vectors (i.e., ticks).14
In the Argentinian endemic area for WNV and SLEV, the ticks Ixodes pararicinus, Ixodes auritulus, Haemaphysalis juxtakochi, Haemaphysalis leporispalustris, Amblyomma dubitatum, Amblyomma ovale, Amblyomma tigrinum, and Amblyomma triste have been reported on wild birds, such as Furnarius rufus, Saltator aurantiirostris, Tarphonomus certhioides, Turdus amaurochalinus, Turdus rufiventris, and Troglodytes aedon15; however, it is uncertain whether these birds play a role in maintaining or disseminating WNV and SLEV. We evaluated the vector competence of three ixodid tick species (A. ovale, A. tigrinum, and Amblyomma tonelliae) for the transmission of WNV and SLEV and evaluated epidemiological implications.
MATERIALS AND METHODS
Ticks.
Larvae of A. ovale, A. tigrinum, and A. tonelliae were obtained from laboratory colonies that were maintained for at most two laboratory generations by feeding on guinea pigs (Cavia porcellus) and New Zealand white rabbits (Oryctolagus cuniculus). The A. ovale colony originated from ticks collected at Peruíbe, São Paulo state, Brazil; the A. tigrinum colony originated from ticks collected at São Roque de Minas, Minas Gerais state, Brazil; and the A. tonelliae colony originated from ticks collected at El Tunal, Salta Province, Argentina. All host animals were obtained from an animal room with no history of tick infestation or tick-borne disease or any contact with acaricides or antibiotic drugs. The breeding methodology for ticks followed the general guidelines described by Guglielmone et al.16
Viral strains.
The WNV E/7229/06 strain, isolated from a dead horse in Buenos Aires Province (Argentina),6 and the SLEV 78V6507 strain, isolated from Culex pipiens quinquefasciatus mosquitoes from Santa Fe Province, Argentina, were used.17 Both viral strains have been passaged three times in suckling mice brain. Viral stocks were prepared as 10% suckling-mouse brains suspension in minimum essential medium with 10% fetal calf serum and 1% gentamycin and were titrated by plaque assay in Vero E6 (WNV) or HuH7.5 (SLEV) cells. The viral titer was expressed as plaque-forming units per milliliter (PFU/mL).
Viremia profile in chicks.
Forty-eight-hour-old chicks (Gallus gallus) seronegative for WNV and SLEV (by plaque reduction neutralization test [PRNT]) were subcutaneously inoculated (five chicks with WNV and five with SLEV) in the ventral region with 0.1 mL of viral suspension containing 300 PFU. All chicks were bled daily from the jugular vein over a 7-day period. Whole blood (0.1 mL) was diluted in 0.9 mL of minimum essential medium with 10% fetal calf serum and 1% gentamycin, and centrifuged at 1,500 g for 15 minutes; the supernatant was stored at −80°C and viremia titers were determined (Figure 1A).
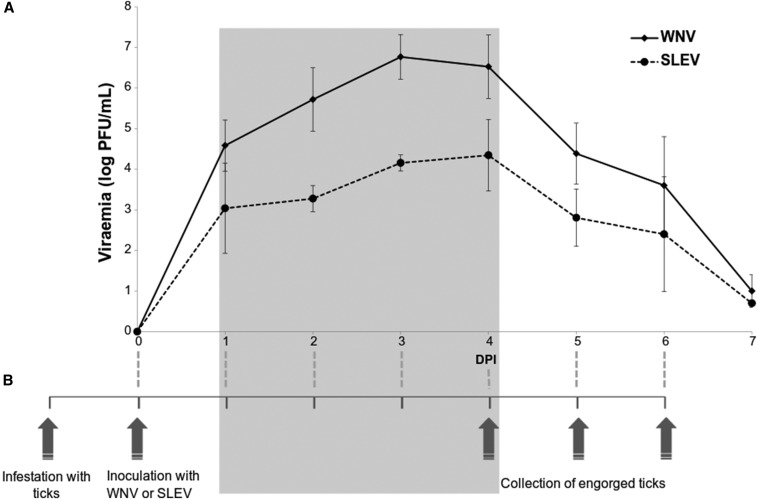
Figure 1.
(A) Viremia profile (mean and standard deviation) for West Nile virus (WNV) and St. Louis encephalitis virus (SLEV) detected in chicks subcutaneously inoculated. (B) Timeline for transmission experiments carried out on this research. The gray box shows that tick feeding occurred during the peak of viremia of the chicks. DPI = days postinoculation.
Tick infection and experimental design.
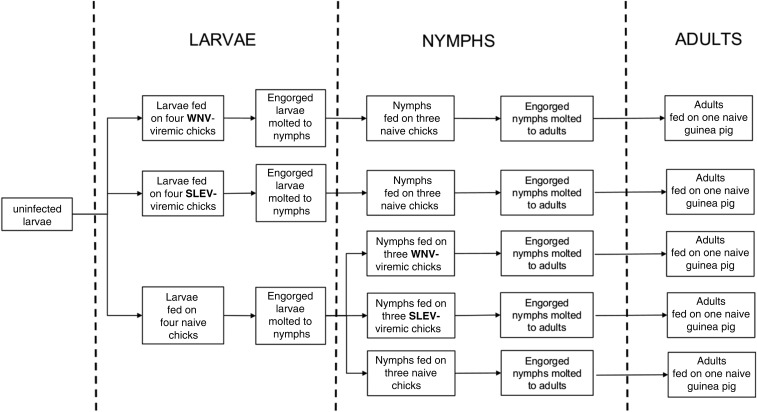
Unfed larvae and nymphs of A. ovale, A. tigrinum, and A. tonelliae were fed on viremic chicks inoculated with WNV or SLEV. Based on the results of chick viremia (Figure 1A), chicks were inoculated with the WNV or SLEV 24 hours after infestation with the ticks; this procedure allowed ticks to feed during the peak of viremia (Figure 1B). Acquisition and transmission trials were conducted with larvae, nymphs, and adults of the same tick generation for the three tick species in parallel. First, uninfected larvae were exposed to viremic (needle-inoculated) or naive (control) chicks. Then, uninfected nymphs were exposed to viremic (needle-inoculated) or naive (control) chicks, and potentially infected nymphs (exposed as larvae on viremic chicks) were fed on naive chicks. Finally, potentially infected adult ticks (exposed as larvae or nymphs on viremic chicks) were fed on guinea pigs (Figure 2).
Figure 2.
Diagram illustrating the sequence of experimental procedures with larvae, nymphs, and adults of each of the three species of ticks in the present study. SLEV = St. Louis encephalitis virus; WNV = West Nile virus.
For larval infestations, 36 chicks (12 chicks per tick species) were used. Uninfected larvae were placed on 24-hour-old chicks. Twenty-four hours after the placement of the larvae, the chicks were inoculated subcutaneously into the ventral region with 0.1 mL of the viral suspension or diluent (medium supplemented with 10% fetal calf serum). For each tick species, four chicks were infected with WNV, four with SLEV, and four were noninfected control. At 48 hours postinoculation, chick blood samples were taken to determine the presence or absence of virus. Naturally detached engorged larvae were recovered and maintained in an incubator at 25°C and 90% relative humidity for molting. On the 15th day of incubation (before beginning the molting process), about one-third of the engorged larvae were stored at −80°C for further testing for the presence of the virus (virus acquisition). The rest of the engorged larvae remained in the incubator for molting to nymphs, which in all cases resulted in > 80% molting success. Nearly 50% of the molted nymphs were used to determine the presence of the virus (transstadial perpetuation) and the remaining were used to infest naive chicks to test vector competence (Figure 2). Serum samples from the animals collected at day 15 after tick infestations were tested for virus and neutralizing antibodies.
For nymphal infestations, 45 chicks, 15 per tick species, were used. For each tick species, five groups of three chicks each were infested: nymphs from uninfected larvae (uninfected control) were fed on naive chicks (control group), on WNV-viremic chicks (WNV group), or on SLEV-viremic chicks (SLEV group). In addition, potentially infected nymphs (previously exposed as larvae by feeding on WNV- or SLEV-viremic chicks) were fed on naive chicks to assess transmission. All unfed nymphs were obtained from experiment I (Figure 2). For each group, the nymphs were placed on three 24-hour-old chicks. Twenty-four hours after the placement of the nymphs, the chicks were inoculated with viral suspension or with diluent (mock infected). At 48 hours postinoculation, the chicks were bled to determine the presence or absence of virus in the blood. Naturally detached engorged nymphs were collected and held in the incubator for molting to adults. At day 20 of incubation (before beginning the molting process), some of the engorged nymphs were placed at −80°C for further testing for the presence of the virus (virus acquisition). The other nymphs remained in the incubator for molting. Nearly 75% of the molted adults were used to determine the existence of transstadial perpetuation and the remaining specimens were used to infest naive guinea pigs (one per tick group) to determine vector competence. Serum samples from the animals were tested for virus and neutralizing antibodies (described in the following paragraph).
Detection of infection in ticks and hosts.
To assess virus acquisition (engorged ticks) and transstadial perpetuation (molted unfed ticks), each tick was thoroughly rinsed with sterile distilled water, triturated in 1.0 mL minimum essential medium supplemented with 10% fetal calf serum and 1% gentamycin, and then centrifuged at 11,400 g for 15 minutes at 4°C. Infectious viral particles were detected by plaque assay in Vero E6 (WNV) or HuH7.5 (SLEV) cells using 100 μL of the tick lysate.
For the vector competence infestations with nymphs and adults that had previously fed on viremic hosts as larvae and nymphs, respectively, the viral infection in chicks (infested with nymphs) and guinea pigs (infested with adults) was determined by plaque assay at 72 hours post-infestation and seroconversion 15 days post-infestation.
Serology.
The presence of neutralizing antibodies was assayed by PRNT. Blood samples were allowed to coagulate at room temperature for 30 minutes, followed by centrifugation to separate the serum. Samples were stored at −20°C and heat inactivated at 56°C for 30 minutes before testing. For PRNT, sera were diluted 1:10 in medium supplemented with 10% fetal calf serum and end point antibody titers were determined using serial 2-fold dilutions. The serum samples that neutralized at least 80% of the inoculated viral plaques were considered positive.
RESULTS
West Nile virus.
Larvae of the three tick species acquired WNV through feeding on viremic chicks, as 18.0%, 3.1%, and 100% of the A. ovale, A. tigrinum, and A. tonelliae engorged larvae, respectively, were shown to contain virus (Table 1). After molting, unfed nymphs derived from these larval batches were tested by plaque assay, which revealed virus transstadial perpetuation in 11.1% and 33.7% of the A. ovale and A. tonelliae nymphs, and none of the A. tigrinum nymphs. None of the chicks exposed to unfed nymphs from these tick groups acquired viral infection, as chicks did not present viremia by plaque assay and did not seroconvert to WNV. Similarly, whereas 3.7% and 0% of the unfed adults of A. tigrinum and A. tonelliae, respectively, derived from larvae exposed to viremic chicks were shown to contain virus, none of the adult ticks from these tick groups transmitted the virus to guinea pigs, which remained virus free by plaque assay and did not seroconvert. No A. ovale adult ticks were available for these tests.
Table 1.
Infection rates of ticks (Amblyomma ovale, Amblyomma tigrinum, and Amblyomma tonelliae) by WNV or SLEV after acquisition feeding experiments on viremic chicks, and vector competence of the post-molted stages of ticks
| Tick species | Virus acquisition* | Transstadial perpetuation† | Vector competence‡ | |||||
|---|---|---|---|---|---|---|---|---|
| Engorged larvae | Engorged nymphs | Unfed nymphs | Unfed adults-L | Unfed adults-N | Nymphs | Adults-L | Adults-N | |
| WNV | ||||||||
| A. ovale | 9/50 (18.0) | 0/5 (0) | 2/18 (11.1) | ND | 0/6 (0) | 0/3 (0) | ND | ND |
| A. tigrinum | 3/96 (3.1) | 0/11 (0) | 0/57 (0) | 1/27 (3.7) | 1/31 (3.2) | 0/3 (0) | 0/1 (0) | 0/1 (0) |
| A. tonelliae | 21/21 (100.0) | 0/4 (0) | 29/86 (33.7) | 0/18 (0) | 3/26 (11.5) | 0/3 (0) | 0/1 (0) | 0/1 (0) |
| SLEV | ||||||||
| A. ovale | 3/28 (10.7) | 0/5 (0) | 3/22 (13.6) | ND | 0/5 (0) | 0/3 (0) | ND | ND |
| A. tigrinum | 0/22 (0) | 0/20 (0) | 12/77 (15.6) | 0/28 (0) | 0/10 (0) | 0/3 (0) | 0/1 (0) | 0/1 (0) |
| A. tonelliae | 5/14 (35.7) | 0/16 (0) | 5/21 (23.8) | 0/20 (0) | 0/21 (0) | 0/3 (0) | 0/1 (0) | 0/1 (0) |
adults-L = adult ticks previously exposed to virus during larval feeding on viremic chicks; adults-N = adult ticks previously exposed to virus during nymphal feeding on viremic chicks; SLEV = St. Louis encephalitis virus; WNV = West Nile virus; ND = no data.
Engorged larvae and nymphs were allowed to feed on viremic chicks and tested by viral infection by plaque assay at 15 days post-host detachment, before molting. Values presented as number of infected ticks/number of tested ticks (% infection).
Unfed nymphs and adults were tested after molting from engorged larvae and adults, respectively, which had fed on viremic chicks. Values presented as number of infected ticks/number of tested ticks (% infection).
Unfed nymphs and adults, previously exposed to acquisition feeding as larvae and nymphs, respectively, on viremic chicks, were allowed to feed on naive hosts (chicks for nymphs and guinea pigs for adults) to evaluate their viral vector competence. Values presented as number of hosts that became viremic or/and seroconverted after infestation/number of infested hosts (each chick or guinea pig was infested with 20 nymphs or eight adult ticks).
Plaque assays did not detect WNV in any of the engorged nymphs of the three tick species that were tested before molting and after feeding on viremic chicks (Table 1). However, in unfed adult ticks derived from these engorged nymphal groups, 0%, 3.2%, and 11.5% of the A. ovale, A. tigrinum, and A. tonelliae ticks, respectively, contained virus. These adult ticks (except for A. ovale, which were not tested) were not competent to transmit WNV to guinea pigs, which remained virus free by plaque assay and also did not seroconvert.
SLEV.
Engorged larvae of A. ovale and A. tonelliae acquired SLEV through feeding on viremic chicks, as 10.7% and 35.7% of them, respectively, were shown to contain virus before molting (Table 1). After molting, unfed nymphs derived from these larval batches were tested by plaque assay, which revealed transstadial virus perpetuation in 13.6% and 23.8% of the A. ovale and A. tonelliae nymphs, respectively. For A. tigrinum, although none of the tested engorged larvae were shown to contain virus, 15.6% of the unfed nymphs contained virus, indicating transstadial perpetuation. Conversely, none of the chicks exposed to unfed nymphs from these tick groups acquired viral infection. Moreover, none of the unfed adult ticks of A. tigrinum and A. tonelliae derived from larvae exposed to viremic chicks were shown to contain virus by plaque assay, and none of the adult ticks from these tick groups transmit the virus to guinea pigs. There were no A. ovale adult ticks available for these tests.
Plaque assay did not detect SLEV in any of engorged nymphs of the three tick species that were tested before molting and after feeding on viremic chicks, nor in unfed adult ticks derived from these engorged nymphal groups (Table 1). In addition, adult ticks of these tick groups (except for A. ovale, which were not tested) were not competent to transmit SLEV to guinea pigs.
Through the study, all ticks and hosts from the uninfected control groups were negative by plaque assay or PRNT for either WNV or SLEV in all trials.
DISCUSSION
In the 1950s, laboratory studies aiming to determine if any tick species might serve as competent vectors for WNV were conducted,18,19 and the unique previous report demonstrating the existence of infection and transstadial transmission of SLEV in ticks was performed using Dermacentor variabilis.20 Since then, the vector competence of several tick species has been evaluated for WNV.21–26 However, no species of South American Ixodidae have been evaluated so far.
In the present study, we demonstrated that larvae and nymphs of A. ovale, A. tigrinum, and A. tonelliae acquired WNV or SLEV after feeding on viremic hosts. Although virus acquisition by feeding and transstadial perpetuation were demonstrated for larvae, and in a lesser extent for nymphs, vector competence was null for both nymphs and adult ticks of the three Amblyomma species examined. Similar results were observed previously with WNV in Ixodes scapularis, Dermacentor andersoni and D. variabilis,22 and Ixodes pacificus in the United States.25 In these studies, immature ticks were infected by WNV after feeding on viremic hosts and retained the virus by transstadial perpetuation, but transmission to hosts was not observed. Lawrie et al.23 demonstrated that Ixodes ricinus became infected with WNV after feeding on infected rodents; however, 30 days after engorgement, they found no evidence of WNV infection. Furthermore, Anderson et al.22 observed that Amblyomma americanum acquired the virus after feeding on hamsters, but no transstadial perpetuation was demonstrated. On three occasions during our assays, the engorged ticks were negative for virus, but the molted ticks of the same challenge were infected. This result suggests a low sample size effect because the number of molted ticks analyzed was greater than the number of engorged ticks.
It is remarkable that these viruses are able to withstand the environmental conditions of the midgut of ticks, which is different from that of mosquitoes. Digestion of blood by ticks is primarily an intracellular process that occurs within the cells of the midgut.27 The susceptibility of arthropod midgut cells is an important determinant of vector competence because it is the primary site for virus replication and represents an intestinal barrier.28 Although the nature of the midgut barrier has not been determined in ticks, it appears to vary among different virus tick systems.29 Similarly, our results suggest that this midgut infection barrier showed variability in larvae and nymphs of A. ovale, A. tigrinum, and A. tonelliae infected by WNV and SLEV, as evidenced for different infection rates in unfed ticks after molting, with a tendency for higher infection rates in A. tonelliae ticks.
Because of the feeding behavior of ticks, the virus must persist from one instar to the next (transstadial perpetuation) to be transmitted to a vertebrate host.28 The existence of transstadial perpetuation may suggest that WNV and SLEV have adaptations that allow them to survive the premolting period, which in the present study lasted 3–4 weeks. There are several strategies adopted by tick-borne viruses that have been adapted to survive the molting period, such as the apparent selection of different specific cell types, tissues, or organs.28,30 This period is important in terms of virus survival because histolytic enzymes and tissue replacement associated with molting provide a potentially hostile environment31; therefore, we can speculate that these viruses could survive the molting period by establishing an infection in at least one cell type that did not undergo histolysis.
During the molting process, salivary glands are reabsorbed and regenerated, so viruses must be able to replicate and to invade new salivary gland cells before being transmitted to the new stage of development.28 Moreover, the timing for viral invasion of new salivary gland cells may be important for successful transmission. Some viruses (tick-borne encephalitis virus and Powassan virus) infect salivary glands before arthropod feeding and are successful transmitted, whereas others (Thogoto virus and Dugbe virus) accumulate in the glands after feeding and, therefore, are not transmitted.32–36 The evidence from our study confirm that in the three Amblyomma tick species analyzed, SLEV and WNV are not transmitted by nymphs nor adults through feeding, and this could be related to the inability of these virus to infect salivary glands before tick feeding.
The results of this study indicate that A. ovale, A. tigrinum, and A. tonelliae are not competent vectors for WNV or SLEV, suggesting that they might not serve as alternative vectors for virus persistence during unfavorable environmental conditions for mosquito vector transmission. However, it is important to mention that these viruses were able to infect these ticks and to survive throughout the molting period, which is important because it increases the adaptive potential. Therefore, if a feeding acquisition occurs on a host in nature, these viruses could persist within these ticks during the unfavorable environmental conditions for mosquito vector transmission. This hypothesis was discussed by Anderson et al.22 for the WNV. They suggest that in northeastern United States, the I. scapularis larvae could be infected by viremic birds. Thus, the virus is able to survive the winter in fed larvae, and if an eventual oral transmission of WNV by ingestion occurs, birds could become infected by ingesting infected ticks. As in the United States, with I. scapularis, the larvae of A. ovale and A. tigrinum (no information for A. tonelliae) feed on birds during unfavorable periods for mosquito transmission (cold and drought).15,37
Vector competence is a result of a complex interaction among virus and arthropod species, and sometimes it would be a very specific relationship. So, we consider that future studies including other tick species (Ixodes and Haemaphysalis) and other sympatric viral strains are required.
Acknowledgments:
We are grateful to Felipe S. Krawczak, João Fábio Soares, and Evelina L. Tarragona for providing the ticks A. ovale, A. tigrinum, and A. tonelliae, respectively, for the present study. We also thank Marta S. Contigiani for her grateful comments. The American Society of Tropical Medicine and Hygiene (ASTMH) assisted with publication expenses.
REFERENCES
- 1.Lanciotti RS, et al. 1999. Origin of the West Nile virus responsible for an outbreak of encephalitis in the northeastern United States. Science 286: 2333–2337. [DOI] [PubMed] [Google Scholar]
- 2.Fang Y, Reisen WK, 2006. Previous infection with West Nile or St. Louis encephalitis viruses provides cross protection during reinfection in house finches. Am J Trop Med Hyg 75: 480–485. [PubMed] [Google Scholar]
- 3.Smithburn KC, Hughes TP, Burke AW, Paul JH, 1940. A neurotropic virus isolated from the blood of a native of Uganda. Am J Trop Med Hyg 20: 471–492. [Google Scholar]
- 4.Chancey C, Grinev A, Volkova E, Rios M, 2015. The global ecology and epidemiology of West Nile virus. Biomed Res Int 2015: 376230. 10.1155/2015/376230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Campbell GL, Marfin AA, Lanciotti RS, Gubler DJ, 2002. West Nile virus. Lancet Infect Dis 2: 519–529. [DOI] [PubMed] [Google Scholar]
- 6.Morales MA, et al. 2006. West Nile virus isolation from equines in Argentina, 2006. Emerg Infect Dis 12: 1559–1561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Diaz LA, et al. 2008. West Nile virus in birds, Argentina. Emerg Infect Dis 14: 689–691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lumsden LL, 1958. St. Louis encephalitis in 1933; observations on epidemiological features. Public Health Rep 73: 340–353. [PMC free article] [PubMed] [Google Scholar]
- 9.Durlach RA, Astarloa L, 1985. Saint Louis meningoencephalitis. Medicina (B Aires) 45: 467–468. [PubMed] [Google Scholar]
- 10.Spinsanti L, et al. 2003. St. Louis encephalitis in Argentina: the first case reported in the last seventeen years. Emerg Infect Dis 9: 271–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Spinsanti LI, et al. 2008. Human outbreak of St. Louis encephalitis detected in Argentina, 2005. J Clin Virol 42: 27–33. [DOI] [PubMed] [Google Scholar]
- 12.Seijo A, et al. 2011. Outbreak of St. Louis encephalitis in the metropolitan Buenos Aires area [in Spanish]. Medicina (B Aires) 71: 211–217. [PubMed] [Google Scholar]
- 13.Diaz LA, Flores FS, Quaglia A, Contigiani MS, 2012. Intertwined arbovirus transmission activity: reassessing the transmission cycle paradigm. Front Physiol 3: 493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mitchell CJ, Francy DB, Monath TP, 1980. Arthropod vectors. Monath TP, ed. St. Louis Encephalitis. Washington, DC: American Public Health Association, 313–379. [Google Scholar]
- 15.Flores FS, Nava S, Batallán G, Tauro LB, Contigiani MS, Diaz LA, Guglielmone AA, 2014. Ticks (Acari: Ixodidae) on wild birds in north-central Argentina. Ticks Tick Borne Dis 5: 715–721. [DOI] [PubMed] [Google Scholar]
- 16.Guglielmone AA, Mangold AJ, García MD, 1991. The life cycle of Amblyomma parvum Aragao, 1908 (Acari: Ixodidae) under laboratory conditions. Exp Appl Acarol 13: 129–136. [DOI] [PubMed] [Google Scholar]
- 17.Mitchell CJ, Monath TP, Sabattini MS, Cropp CB, Daffner JF, Calisher CH, Jakob WL, Christensen HA, 1985. Arbovirus investigations in Argentina, 1977–1980. II. Arthropod collections and virus isolations from Argentine mosquitoes. Am J Trop Med Hyg 34: 945–955. [PubMed] [Google Scholar]
- 18.Hurlbut HS, 1956. West Nile virus infection in arthropods. Am J Trop Med Hyg 5: 76–85. [DOI] [PubMed] [Google Scholar]
- 19.Hurlbut HS, Rizk F, Taylor RM, Work TH, 1956. A study of the ecology of West Nile virus in Egypt. Am J Trop Med Hyg 5: 579–620. [DOI] [PubMed] [Google Scholar]
- 20.Blattner RJ, Heys FM; Technical Assistance of Margaret B. McDonald , 1944. Blood-sucking vectors of encephalitis: experimental transmission of St. Louis encephalitis (Hubbard strain) to white Swiss mice by the American dog tick, Dermacentor variabilis say. J Exp Med 79: 439–454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Abbassy MM, Osman M, Marzouk AS, 1993. West Nile virus (Flaviviridae: Flavivirus) in experimentally infected argas ticks (Acari: Argasidae). Am J Trop Med Hyg 48: 726–737. [DOI] [PubMed] [Google Scholar]
- 22.Anderson JF, Main AJ, Andreadis TG, Wikel SK, Vossbrinck CR, 2003. Transstadial transfer of West Nile virus by three species of ixodid ticks (Acari: Ixodidae). J Med Entomol 40: 528–533. [DOI] [PubMed] [Google Scholar]
- 23.Lawrie CH, Uzcategui NY, Gould EA, Nuttall PA, 2004. Ixodid and argasid tick species and West Nile virus. Emerg Infect Dis 10: 653–657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Formosinho P, Santos-Silva MM, 2006. Experimental infection of Hyalomma marginatum ticks with West Nile virus. Acta Virol 50: 175–180. [PubMed] [Google Scholar]
- 25.Reisen WK, Brault AC, Martinez VM, Fang Y, Simmons K, Garcia S, Omi-Olsen E, Lane RS, 2007. Ability of transstadially infected Ixodes pacificus (Acari: Ixodidae) to transmit West Nile virus to song sparrows or western fence lizards. J Med Entomol 44: 320–327. [DOI] [PubMed] [Google Scholar]
- 26.Kokonova MS, Borisevich SV, Grabarev PA, Bondarev VP, 2013. Experimental assessment of the possible significance of argasid ticks in preserving the natural foci of West Nile virus infection [in Russian]. Med Parazitol (Mosk) 2: 33–35. [PubMed] [Google Scholar]
- 27.Sonenshine DE, 1991. Biology of Ticks, 1st edition. New York, NY: Oxford University Press. [Google Scholar]
- 28.Labuda M, Nuttall PA, 2004. Tick-borne viruses. Parasitology 129: S221–S245. [DOI] [PubMed] [Google Scholar]
- 29.Steele GM, Nuttall PA, 1989. Difference in vector competence of two species of sympatric ticks, Amblyomma variegatum and Rhipicephalus appendiculatus for Dugbe virus (Nairovirus: Bunyaviridae). Virus Res 14: 73–84. [DOI] [PubMed] [Google Scholar]
- 30.Davies CR, Jones LD, Nuttall PA, 1986. Experimental studies on the transmission cycle of Thogoto virus, a candidate orthomyxovirus, in Rhipicephalus appendiculatus ticks. Am J Trop Med Hyg 35: 1256–1262. [DOI] [PubMed] [Google Scholar]
- 31.Balashov JC, 1998. Ixodid Ticks—Parasites and Vectors of Diseases. Sankt-Peterburg, Russia: Nauka. [Google Scholar]
- 32.Rehacek J, 1965. Development of animal viruses and rickettsiae in ticks and mites. Annu Rev Entomol 10: 1–24. [Google Scholar]
- 33.Chernesky MA, Mclean DM, 1969. Localization of Powassan virus in Dermacentor andersoni ticks by immunofluorescence. Can J Microbiol 15: 1399–1408. [DOI] [PubMed] [Google Scholar]
- 34.Booth TF, Davies CR, Jones LD, Staunton D, Nuttall PA, 1989. Anatomical basis of Thogoto virus infection in BHK cell culture and in the ixodid tick vector, Rhipicephalus appendiculatus. J Gen Virol 70: 1093–1104. [DOI] [PubMed] [Google Scholar]
- 35.Booth TF, Steele GM, Marriott AC, Nuttall PA, 1991. Dissemination, replication, and trans-stadial persistence of Dugbe virus (Nairovirus, Bunyaviridae) in the tick vector Amblyomma variegatum. Am J Trop Med Hyg 45: 146–157. [DOI] [PubMed] [Google Scholar]
- 36.Kaufman WR, Nuttall PA, 2004. Rhipicephalus appendiculatus (Acari: Ixodidae): dynamics of Thogoto virus infection in female ticks during feeding on Guinea pigs. Exp Parasitol 104: 20–25. [DOI] [PubMed] [Google Scholar]
- 37.Nava S, Mangold AJ, Guglielmone AA, 2009. Seasonal distribution of larvae and nymphs of Amblyomma tigrinum Koch, 1844 (Acari: Ixodidae). Vet Parasitol 166: 340–342. [DOI] [PubMed] [Google Scholar]